|
|
Post by Admin on Oct 8, 2018 18:19:36 GMT
To infer sex-specific admixture rates and compare potential migration models, we estimated ancestry proportions on the X chromosome and autosomes separately, with a model-based clustering algorithm (33), using the ancient genomes as proxies for the ancient source groups in our population model and using supervised clustering (Materials and Methods, Fig. 2, and SI Appendix, Figs. S1 and S2 and Tables S1–S7). For an admixture process with equally many males and females contributing, the ratio of mean X-chromosomal admixture to mean autosomal admixture is expected to be 1. An admixture process with more contributing males leads to a reduction of the migrating population’s ancestry on the X chromosome compared with the autosomes.  Fig. 2. Comparisons of estimated X and autosomal ancestry on the basis of model-based supervised clustering. (A) Early/middle Neolithic Europeans (CE). (B) Late Neolithic/Bronze Age Europeans (BA). Individuals are ordered by X-chromosomal ancestry, with corresponding autosomal ancestry for the same individual shown below. Clustering results by individual are presented in SI Appendix, Table S1. (C) Histograms of the ratio of the mean across individuals of X-chromosomal ancestry to the mean across individuals of autosomal ancestry for 100 autosomal resampled estimates using random sets of SNPs equal in size to the set of X-chromosomal SNPs for the corresponding population (Materials and Methods). Colors for all panels correspond to ancestry groups given in Fig. 1. We additionally considered the fraction of individuals in the admixed population with higher X-chromosomal than autosomal ancestry. This measure is indicative of sex bias, with less emphasis on the exact values of the ancestry proportions. Excluding three individuals with 100% ancestry estimated to be from Anatolian-related populations on both the X and autosomes, nine of 17 individuals have higher X than autosomal ancestry (P = 0.500, binomial test).  Principal component analysis of ancient genomes. Notably, the four middle Neolithic individuals (SI Appendix, Table S1) have higher HG ancestry than earlier CE individuals, consistent with a previously described resurgence of HG ancestry during the middle Neolithic (5, 34). The ratio of the mean X to the mean autosomal ancestry for this group of four samples is 0.792/0.802=0.988, supporting no sex bias in farming contributions to CE individuals. Similarly, we see a significant relationship between sample age and ancestry when fit to a linear model (Materials and Methods), although the similarity of X and autosomal ancestry holds over time (SI Appendix, Fig. S1A). We find no statistical support for differences in X and autosomal ancestry; however, we cannot exclude low levels of sex-specific mating between early farmers and hunter-gatherers. Therefore, we evaluated the magnitude of differences in male and female contributions that would be consistent with observed X-to-autosomal ancestry ratios. We determined this range of sex bias values by simulating ancestry under a mechanistic admixture model, including genetic drift and sampling at specified sample sizes (17, 35, 36) (Materials and Methods and Fig. 3A). Even for a small admixed population, the largest bias consistent with the observed X and autosomal ancestries is less than 1.2 males for every female, with a median over 1,000 simulations of 1.07.  Pairwise outgroup f3 statistics. |
|
|
|
Post by Admin on Oct 9, 2018 18:06:06 GMT
 Fig. 3. Estimated levels of sex bias during the Neolithic transition and Pontic Steppe migration. (A) Neolithic transition. The range of sex bias, measured as the ratio of males to females from a source population, that is consistent with the observed ratio of X and autosomal ancestries (Materials and Methods). Total contributions from the source population, the fraction of admixed individuals with a parent from that source population, are specified based on autosomal ancestry as 0.913 from AF and 0.087 from HG. Lines indicate that the observed ratios of X to autosomal ancestry in our dataset were present in the middle 50% (black) or middle 80% (gray) of 1,000 simulated admixed populations for specified CE population sizes. (B) Pontic Steppe migration. Under a model of constant admixture over time, the fraction of the total contribution of genetic material originating from males for each source population: CE and SP. Contributions are estimated from the migration parameter sets that have the smallest 0.1% Euclidean distance between observed and model-calculated ancestries. (C) Schematic of sex-specific migrations during the early Neolithic and later Neolithic/Bronze Age. Female contributions in are shown in red, and male contributions are shown in blue. Parameters are estimated under a single pulse migration model from Anatolia and under a constant migration model for the Pontic Steppe migration. The total contribution of each population is the average of female and male contributions from that source. Based on the slightly larger X than autosomal ancestry observed for HG ancestry, under the simulation framework, we estimate a median of 1.91 females for every male from the HG population to the early CE population. The signal of female bias in contributions from HG to CE populations might be caused by a male-biased inheritance structure in the new farming population; that is, it is possible that the migration from Anatolia involved substantial contributions from both men and women, but once in central Europe, a shift to patrilocality might have made absorption of local HG females easier than absorption of HG males. However, the absolute difference between estimated male and female contributions is small (∼0.06). Correspondingly, differences in the numbers of female and male migrants would be small or are potentially a result of sampling. Considering these analyses together, we find no statistical support for a male-biased migration from Anatolia. Only a small range of possible sex bias is consistent with the data; however, owing to the small total contribution from the HG population, we see female-biased contributions from the HG to CE populations (Fig. 3A). |
|
|
|
Post by Admin on Oct 10, 2018 18:19:29 GMT
Pontic-Caspian Steppe Migration. We next considered female and male migration histories during the late Neolithic/Bronze Age migration from the Pontic-Caspian Steppe (Fig. 1). In contrast to the CE population during the early Neolithic expansion from Anatolia, we find a strikingly lower distribution of SP ancestry on the X chromosome than the autosomes (in accordance with FST results; Fig. 2 and SI Appendix, Fig. S2), suggesting extreme male-biased migration from SP during the late Neolithic/Bronze Age migration from the Pontic-Caspian Steppe. Using an approach that is similar to the approach used for the early Neolithic migration event, the ratio of mean X-chromosomal SP ancestry to mean autosomal SP ancestry in the BA population is 0.366/0.618=0.592 0.634/0.382=1.6600.634/0.382=1.660. Of 16 admixed BA individuals, 12 have more SP ancestry on the autosomes than on the X chromosome (binomial test, P = 0.038). Similarly, the distribution of P-values of the Wilcoxon signed-rank test comparing the estimated X-chromosomal ancestries with the autosomal ancestries in each of 100 resamples of autosomal SNPs is highly skewed toward zero, with a median of P = 0.02 (Materials and Methods and SI Appendix, Fig. S3). To interpret the values of sex-specific admixture that can produce the observed ratio of X to autosomal SP ancestry of about 0.6, we considered four models for the admixture process. The first model is a single admixture event, in which an SP population quickly mixes with central European farmers, with no further migration from either population to the admixed BA population. Under this model, however, the level of sex bias is too high to have been produced by a single admixture event; no solution for the female and male migration rates exists within the possible admixture contribution range from 0 to 1 (Materials and Methods). In other words, in a pulse migration and admixture scenario in a single generation, even a male-only migration event is not extreme enough to generate the observed X-to-autosome bias in the data. Ongoing male migration from the steppe over multiple generations is therefore required to explain observed patterns of X and autosomal ancestry. 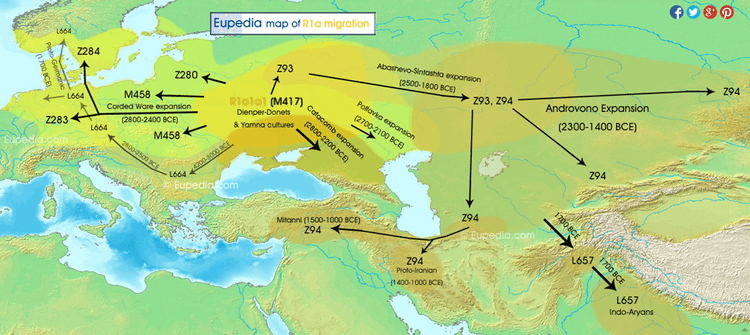 We therefore considered a model of constant contributions over time from the SP population and early Neolithic farmers (CE). We follow the method of Goldberg and Rosenberg (17), comparing expected X and autosomal ancestry (equations 5, 17, and 18 of ref. 17 and equation 30 of ref. 35) with observed ancestry in our data over a grid of possible parameter values. We present results from the 0.1% of parameter sets closest to observed data using a Euclidean distance between model-based and observed population mean ancestries on the X and autosomes (Materials and Methods). Other cutoffs (0.5%, 1%, and 5%) produced similar trends. SI Appendix, Fig. S4 plots the range of sex-specific contributions from the SP and CE populations that produce estimates close to the estimates observed in the BA population. Males from the steppe and central European females show substantial ongoing migration, with continuing admixture rates of almost 1/2; that is, almost half of the male parents in each generation of BA individuals are new migrants from the SP population. Females from the steppe and early Neolithic European males, however, are estimated to have contributed negligibly to the BA population. Fig. 3B plots the proportional contribution of males from each source population, with a median of about 94% of SP ancestry in the BA population coming from male SP migrants and all local CE ancestry originating in CE females. This result corresponds to ∼14 male migrants for every female migrant from the steppe contributing to the ancestry of the BA population. Considering the smallest 0.5%, 1% , and 5% of Euclidean distances instead, this ratio is about 8.5, 7.5, and 5.1, respectively, males per female migrating from the steppe. These estimates are similar to estimates from modern Y-chromosome data, suggesting a reduction in the male effective population size by more than fivefold about 5,000 y ago (28). The signature of X-chromosomal to autosomal ancestry is driven by the last few generations of admixture. Testing other models of time-dependent admixture, with the contributions from one or both of the source populations increasing or decreasing over time, we find that the data fit model-based estimates approximately equally well when the admixture contributions at the last few generations are similar to the admixture contributions estimated from a constant admixture model (Materials and Methods).  The signal of a large male bias holds when analyzing late Neolithic Corded Ware individuals and later Bronze Age Unetice individuals separately, with mean X-to-autosomal ancestry ratios in the two groups of 0.716 and 0.474, respectively. Ancestry and sex bias do differ between the groups, with a larger male bias and lower SP ancestry for the later Unetice, although the trend is not statistically significant (SI Appendix, Fig. S1B). Individuals from Bell Beaker archeological sites, a culture that overlapped with Corded Ware and Unetice but occurred over a wider geographic scale, show levels of X and autosomal ancestry suggestive of overall ancestry contributions and levels of sex bias that are similar to Corded Ware and Unetice, with mean X and autosomal ancestry of 0.28 and 0.56, respectively (SI Appendix, Table S7). The signal of male-biased contributions from SP to BA over time is consistent with an admixture scenario in which a massive male-biased migration from the steppe initially looks to local European farmer females for wives, and with a paternal mode of inheritance, the BA population disproportionately absorbs females from local “unadmixed” farmers. Admixture from the steppe population continues over time, although mainly men migrate, perhaps expanding using the male-dominated modes of horses and wagons (24, 30). PNAS March 7, 2017 114 (10) 2657-2662 |
|
|
|
Post by Admin on Feb 9, 2019 20:50:31 GMT
The surprising finding, published online February 4 in Nature Communications, raises novel questions about a pivotal time when widespread foraging and farming populations interacted in Eurasia’s Caucasus region. Those exchanges presumably sparked the geographic spread of metalworking, the wheel and wagon, and Indo-European languages still spoken in much of the world. Archaeologists have often assumed that, as early as around 5,600 years ago, Caucasus farmers known as the Maykop migrated north in big numbers, bringing metalworking and early Indo-European tongues to herders who roamed grasslands on the edge of the region. In that scenario, this cultural exchange led steppe herders to develop a horse-and-wagon lifestyle that the nomads later transported to Europe and Asia, along with Indo-European languages, starting about 5,000 years ago (SN: 11/25/17, p. 16). Researchers call those mobile herders Yamnaya people.  An ancient DNA analysis unexpectedly found signs of mating more than 5,000 years ago between western Asian Yamnaya herders and European farmers, possibly from the Globular Amphora Culture. In another surprise, Maykop farmers thought by many researchers to have dramatically influenced Yamnaya culture left no genetic mark on the herders. The dotted lines represent the suspected spheres of influence exercised by the Globular Amphora Culture and the Maykop people in Yamnaya territory. Yamnaya DNA shows signs of a shared ancestry only with eastern European farmers, not the Maykop people. The genetic analysis, led by population geneticist Chuan-Chao Wang of Xiamen University in China and molecular anthropologist Wolfgang Haak of the Max Planck Institute for the Science of Human History in Jena, Germany, provides the best look to date at Yamnaya herders’ genetic history. The scientists analyzed sets of inherited alterations in the DNA of 45 individuals, including four Yamnaya and 12 Maykop, excavated from Caucasus and steppe graves dating to between 6,500 and 3,500 years ago. Comparisons were made to previously extracted DNA from other ancient Europeans, Asians and Native Americans.  A majority of Yamnaya ancestry came from Caucasus-based hunter-gatherers and a minority of Yamnaya ancestry — between 10 and 18 percent — was inherited from eastern European farmers, the scientists estimate. Those farmers may have belonged to Europe’s more than 5,000-year-old Globular Amphora Culture, named for its globe-shaped pottery. These results indicate that, well before Yamnaya herders made a big-time move to Europe, “there was a sphere of interaction in eastern Europe between people of otherwise very different genetic backgrounds,” Haak says. Maykop people, on the other hand, inherited about half of their DNA from Anatolian farmers, who inhabited what’s now Turkey, Haak and his colleagues report. That finding further underscores the genetic separation of Maykop farmers from Yamnaya herders, who don’t share any DNA with Anatolian cultivators.  Evidence of scant mating between hill-dwelling Maykop farmers and steppe-dwelling Yamnaya herders is “a big surprise,” says archaeologist Volker Heyd of the University of Helsinki, who did not participate in the study. Eastern Europe’s Globular Amphora Culture looks like a good candidate for having mated to some extent with Yamnaya people more than 5,000 years ago, Heyd adds. Migrations of some Maykop into Yamnaya territory, accompanied by the transfer of knowledge and language, still happened, Wang’s team suspects. Occasional migrations north through the Caucasus to Yamnaya grasslands fits a scenario in which the ancient homeland of Indo-European language lay among Anatolian farmers, the researchers speculate. If they’re right, they have resolved one of the thorniest issues in the study of languages. But the long-debated origins of Indo-European tongues remain uncertain. Maykop people excavated in Yamnaya territory came from a small, isolated population that shows no signs of herder ancestry, contends archaeologist David Anthony of Hartwick College in Oneonta, N.Y. “It just emphasizes that Maykop people chose not to mate with Yamnaya or pre-Yamnaya people.” Without regular marriages across the two cultures, Maykop people would not have transferred their language to the Yamnaya, Anthony contends. He considers it likely that Indo-European precursor languages originated among steppe herders. |
|
|
|
Post by Admin on Feb 11, 2019 18:14:51 GMT
 Alternatively, it could be plausible that R1b1 was native to the Pontic-Caspian steppes. A male from the Mesolithic Samara culture (5,650–5,555 BCE) belonged to R1b1, which was the earliest documented ancient sample of this haplogroup discovered to date (Haak et al. 2014). As discussed in the Maycop thread, there was cultural interaction between the steppe herders and the Maykop people from the Near East (Wang et al. 2018) and the steppe herders could have adapted to the Maykop culture, thus abandoning their previous hunter-gatherer lifestyle. In this complex civilizing process, dynastic marriages could have played a part as some Yamnaya individuals were buried in Maykop kurgans and Yamnaya chieftains could have intermarried with Maykop chieftains. Second, our results reveal that the Greater Caucasus Mountains were not an insurmountable barrier to human movement in prehistory. Instead the foothills to the north at the interface of the steppe and mountain ecozones could be seen as a transfer zone of cultural innovations from the south and the adjacent Eurasian steppes to the north, as attested by the archaeological record. The latter is best exemplified by the two Steppe Maykop outlier individuals dating to 5100-5000 yBP/3100-3000 calBCE, which carry additional Anatolian farmer-related ancestry likely derived from a proximate source related to the Caucasus cluster. We could show that individuals from the contemporaneous Maykop period in the piedmont region are likely candidates for the source of this ancestry and might explain the regular presence of ‘Maykop artefacts’ in burials that share Steppe Eneolithic traditions and are genetically assigned to the Steppe group. Hence the diverse ‘Steppe Maykop’ group indeed represents the mutual entanglement of Steppe and Caucasus groups and their cultural affiliations in this interaction sphere. 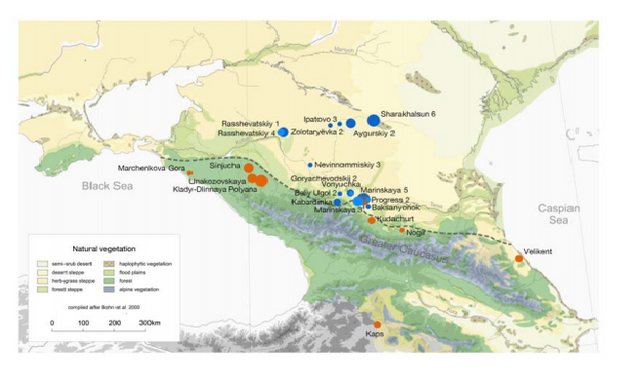 Wang et al. (2018) are not sure about the exact source of Near Eastern admixture in the Yamnaya (16%) due to the limits of their data and the paper didn't specifically mention how the two populations are genetically related to each other. The exact geographic and temporal origin of this Anatolian farmer-related ancestry in the North Caucasus and later in the steppe is difficult to discern from our data. Not only do the Steppe groups vary in their respective affinity to each of the two, but also the Caucasus groups, which represent potential sources from a geographic and cultural point of view, are mixtures of them both23. Another recent study by Jones et al. (2015) found that the Yamnaya are estimated to owe half of their ancestry to CHG-linked sources, which may be linked to the Maykop culture. Probably future studies will clarify this issue when the full Y-DNA profile of the Maykop culture is made available.  The Yamnaya were semi-nomadic pastoralists, mainly dependent on stock-keeping but with some evidence for agriculture, including incorporation of a plow into one burial26. As such it is interesting that they lack an ancestral coefficient of the EF genome (Fig. 1b), which permeates through western European Neolithic and subsequent agricultural populations. During the Early Bronze Age, the Caucasus was in communication with the steppe, particularly via the Maikop culture27, which emerged in the first-half of the fourth millennium BC. The Maikop culture predated and, possibly with earlier southern influences, contributed to the formation of the adjacent Yamnaya culture that emerged further to the north and may be a candidate for the transmission of CHG ancestry. In the ADMIXTURE analysis of later ancient genomes (Fig. 1b) the Caucasus component gives a marker for the extension of Yamnaya admixture, with substantial contribution to both western and eastern Bronze Age samples. However, this is not completely coincident with metallurgy; Copper Age genomes from Northern Italy and Hungary show no contribution; neither does the earlier of two Hungarian Bronze Age individuals. The separation between CHG and both EF and WHG ended during the Early Bronze Age when a major ancestral component linked to CHG was carried west by migrating herders from the Eurasian Steppe. The foundation group for this seismic change was the Yamnaya, who we estimate to owe half of their ancestry to CHG-linked sources. These sources may be linked to the Maikop culture, which predated the Yamnaya and was located further south, closer to the Southern Caucasus. Through the Yamanya, the CHG ancestral strand contributed to most modern European populations, especially in the northern part of the continent. 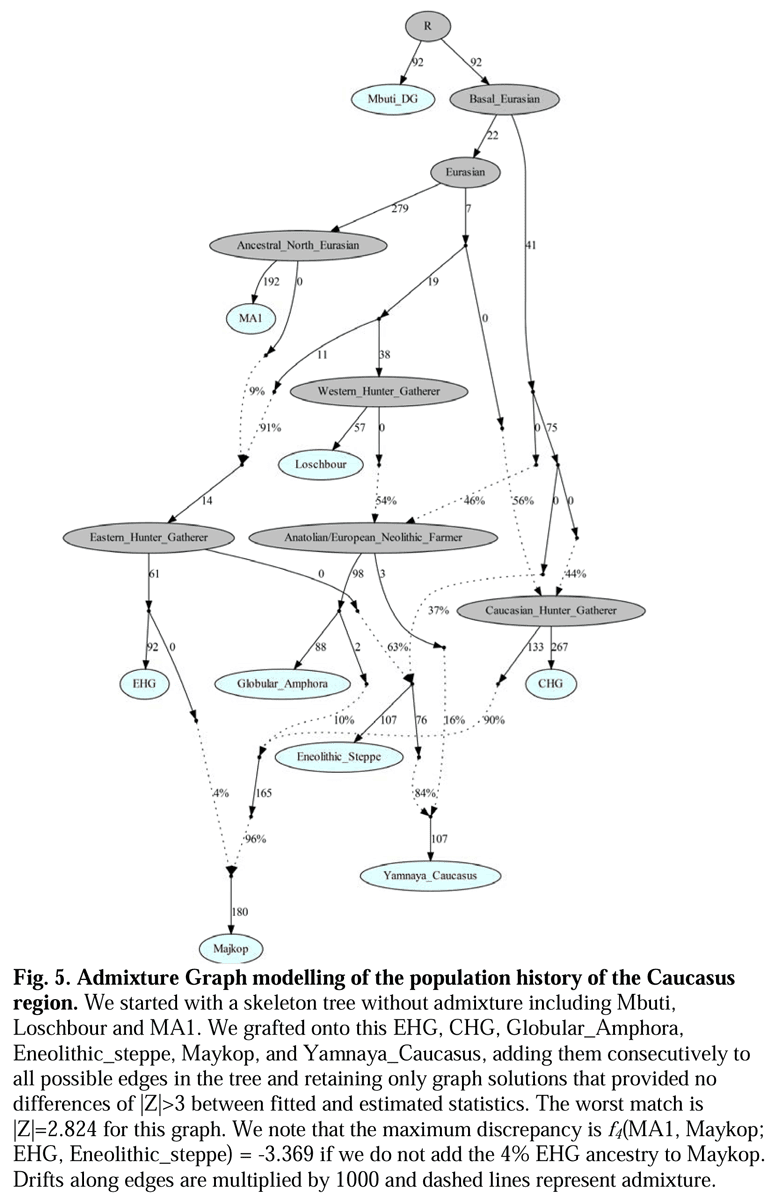 This study by Wang et al. (2018) is significant as a major full-length study on the Maykop culture in human genetics. Prior to this study, we only knew about the Maykop culture's mtDNA haplogroups such as T2b, N1b1, U8b1a2 and V7 (Sokolov et al. 2016) , but we are making progress. We could show that individuals from the contemporaneous Maykop period in the piedmont region are likely candidates for the source of this ancestry and might explain the regular presence of ‘Maykop artefacts’ in burials that share Steppe Eneolithic traditions and are genetically assigned to the Steppe group. Hence the diverse ‘Steppe Maykop’ group indeed represents the mutual entanglement of Steppe and Caucasus groups and their cultural affiliations in this interaction sphere.
Sample Site Age, BP Culture mtDNA Y-DNA
AY2001.A0101.TF1.1 Aygurskiy 2 5271.5 Steppe Maykop T2e
AY2003.A0101.TF1.1 Aygurskiy 2 5455.5 Steppe Maykop H2a1
MK3003.A0101 Marinskaya 3 4476.5 Catacomb U4a2
MK5012.A0101 Marinskaya 5 4663.5 Catacomb U5a1b1e ?
RK4002.B0101 Rasshevatskiy 4 4610.0 Catacomb U4d3 R1b1a2
RK4001.A0101 Rasshevatskiy 4 4277.0 Catacomb U5a1i R1b1a2
SA6003.B0101 Sharakhalsun 6 4292.5 Catacomb U2e3a R1b1a2
I6278 Shepsi 5200.0 Dolmen BA T1a2 ..
I6281 Shepsi 5200.0 Dolmen BA U2e1 ..
I2051 Marchenkova Gora, D13 3260.0 Dolmen LBA H6a1a2a J
I2055 Unakozovskaya 6533.0 Eneolithic Caucasus R1a J
I2056 Unakozovskaya 6477.5 Eneolithic Caucasus R1a J2a
I1722 Unakozovskaya 6403.5 Eneolithic Caucasus R1a
PG2001 Progress 2 6207.0 Eneolithic steppe I3a R1b1
PG2004 Progress 2 6090.0 Eneolithic steppe H2 R1b1
VJ1001 Vonyuchka 1 6242.0 Eneolithic steppe T2a1b
ARM001.A0101 Kaps 5329.5 Kura-Araxes R1a1
ARM002.A0101; ARM003 Kaps 5148.0 Kura-Araxes K3 G2b
VEK006.A0101 Velikent 4850.0 Kura-Araxes U4a2
VEK007.A0101; VEK009 Velikent 4850.0 Kura-Araxes U4a2 J1
VEK008.A0101 Velikent 4850.0 Kura-Araxes U4a2 ?
MK5008.B0101 Marinskaya 5 5185.5 Late Maykop T1a2 ?
MK5004 Marinskaya 5 5171.0 Late Maykop T2al L
MK5001 Marinskaya 5 5141.5 Late Maykop K1a4 L
SIJ003.A0101 Sinyukha 5174.0 Late Maykop U4c1
SIJ002.A0101 Sinyukha 5173.5 Late Maykop U4c1 L
SIJ001.A01(SA6002.A01) Sinyukha 5125.5 Late Maykop U4c1
KBD001 Kabardinka 4036.5 Late North Caucasus I4a R1b1a2
KBD002.A0101 Kabardinka 4057.0 Late North Caucasus W1+119
NV3001 Nevinnomiskiy 3 3970.5 Lola R1b Q1a2
I1720 Baksanenok 5300.0 Maykop HV ?
MK5007.B0101 Marinskaya 5 5455.0 Maykop U5a1b1
OSS001.A0101 Nogir 3 5570.0 Maykop J2a1
I6268 Klady 5564.0 Maykop Novosvobodnaya R1a J2a1
I6267 Klady 5438.0 Maykop Novosvobodnaya T2c1
I6270 Klady 5434.0 Maykop Novosvobodnaya U1b ?
I6266 Klady 5200.0 Maykop Novosvobodnaya X2f J2a1
I6272 Dlinnaya Polyana 5200.0 Maykop Novosvobodnaya U1b1 G2a2a
KDC001.A0101 Kudachurt 3823.5 MBA North Caucasus X2i J2b
KDC002.A0101 Kudachurt 3734.5 MBA North Caucasus HV1a1
BU2001.A0101 Beliy Ugol 2 4674.0 North Caucasus R1b1a2a2
GW1001.A0101 Goryachevodskiy 2 4726.0 North Caucasus U2e1b R1b1a2a2
I1723 Goryachevodskiy 2 4702.0 North Caucasus U5b2a1a R1b1a1a2a
LYG001.A0101 Lysogorskaya 6 4672.0 North Caucasus H13a1a2 R1b1a2
MK5009.A0101 Marinskaya 5 4710.0 North Caucasus R1a1a R1b1a2
PG2002.A0101 Progress 2 4362.5 North Caucasus U1a1a3
RK1003.C0101 Rasshevatskiy 1 4750.5 North Caucasus R1a1a
SA6001.A0101 Sharakhalsun 6 5444.0 Steppe Maykop U7b
SA6004 Sharakhalsun 6 5170.5 Steppe Maykop U7b Q1a2
IV3002.A0101 Ipatovo 3 5206.5 Steppe Maykop outlier X1'2'3 ?
SA6013.B0101 Sharakhalsun 6 5180.0 Steppe Maykop outlier I5b R1
RK1007.A0101 Rasshevatskiy 1 5123.0 Yamnaya Caucasus T2a1
RK1001.C0101 Rasshevatskiy 1 4726.0 Yamnaya Caucasus U5a1d R1b1a2
SA6010.A0101 Sharakhalsun 6 4731.5 Yamnaya Caucasus U5a1g ?
ZO2002.C0101 Zolotarevka 2 4850.0 Yamnaya Caucasus U5a1+@16192
Marinskaya 3 and Marinskaya 5 are closely associated with Maykop people as these catacombs are located at the heart of the Maykop culture. The last Marinskaya 5 specimen (MK5009.A0101) belonged to Y-DNA haplogroup R1b1a2 and two other unassigned specimens may have had R1b1a2 as well, given the cultural and genetic continuity in the area. Rasshevatskiy 4 and Sharakhalsun 6 (R1b1a2) were also originally Maykop burials, which were later utilized by Yamnaya people who added extra graves. During the 3rd millennium, early Yamnaya groups used the Maykop mound and added several graves to honor their ancestors. Without regular marriages across the two cultures, Maykop people would not have transferred their language to the Yamnaya. |
|
















