|
|
Post by Admin on Apr 3, 2017 20:10:19 GMT
 The Eurasian steppes extend over more than 8000 km from Ukraine to Mongolia. This immense territory can be divided into several zones according to climate and vegetation. Indeed, there is a gradient of increasingly arid lands between the north and the south that separates the wooded steppe, the grassy steppe and the semi-desert steppe. Only the mountain massif of the Altai forms a geological barrier high of more than 4000 meters but which nevertheless remains crossable in many places. It spans over 2000 km between Russia, Kazakhstan, Mongolia and Xinjiang in northwestern China. Modern man has been present in the Altai region for over 40,000 years. In the Bronze Age, the cultural sequence began in the middle of the 4th millennium BC. JC, with the appearance of the culture of Afanasievo. Archaeological and anthropological studies have suggested a link between this culture and the Yamnaya culture of the steppes of Eastern Europe. The culture of Afanasievo is preceded by a very little documented culture: the Bol'shemysskaya culture. In the Minusinsk basin, the Okunevo culture follows the Afanasievo culture. Although similar to the Afanasievo culture, anthropological studies indicate that the population is more Mongoloid than its predecessor. This crop coexisted with the Ekunino crops in the foothills of Altai and Chemurchek in northwestern Mongolia. These crops were then replaced by the culture of Andronovo, then by the cultures of Karasuk , Munkh Khairkan and Sagsai at the end of the Bronze Age.  During this period, the domestication of the horse as well as the use of the tanks, allowing to be more mobile, will participate in the development and the diffusion of these cultures along the Eurasian steppes. Clémence Hollard published his thesis in 2014: Population of the south of Siberia and Altai in the Bronze Age: contribution of the paleogenetic. She analyzed the DNA of 69 samples from different Bronze Age sites in the Altai region:  |
|
|
|
Post by Admin on Apr 4, 2017 19:59:56 GMT
 In the figure above, the numbers correspond to the following crops: Munkh Khairkan and Sagsai at the end of the Bronze Age. During this period, the domestication of the horse as well as the use of the tanks, allowing to be more mobile, will participate in the development and the diffusion of these cultures along the Eurasian steppes. She analyzed the DNA of 69 samples from different Bronze Age sites in the Altai region: In the figure above, the numbers correspond to the following crops: Will participate in the development and spread of these crops along the Eurasian steppes. Afanasievo (3500 to 2600 BC) and Okunevo (2300 to 1800 BC) Elunino (2300 to 1700 BC) Bol'shemysskaya (4th millennium BC) and Afanasievo (3500-2600 BC). Chemurchek (2500 to 2300 BC) Sagsai (1400 to 1100 BC) Sagsai (1400 to 900 BC) and Afanasievo (2740 BC). Chemurchek (2300 to 1800 BC) Munkh-Khairkhan (1700-1400 BC) Munkh-Khairkhan (1700-1400 BC) Chemurchek (24300 to 20800 BC) Baitag (1200 to 900 BC)  Genetic analyses performed on these samples were sex determination, autosomal STRs, HVR1 mitochondrial region sequencing, mitochondrial coding region SNPs test to discriminate major haplogroups, 17 STRs assay of the chromosome Y, the 27 Y SNPs to discriminate the main haplogroups, and the assay of 28 autosomal SNPs to determine phenotypic characteristics: hair and eye color. Regarding the oldest populations: Bol'shemysskaya and Afanasievo, the individuals belong to the haplogroup R1b of the Y chromosome, Except for one individual from Mongolia of the haplogroup Q. Among the R1b individuals, two were tested positive for M269. Moreover, analysis of the STR haplotypes indicates that these individuals R1b group with individuals R1b-L23. These results are similar to previous studies which showed that individuals of the Yamnaya population of the Pontic Steppes were also of the haplogroup R1b-L23. The mitochondrial haplogroups of the skeletons of these populations are half western: H and U, half oriental: C. Finally, the results of autosomal STR markers show that the Afanasievo individuals are of western origin, close to the present Europeans.  For the next phase, individuals in the Okunevo culture show an evolution of their haplogroups of the Y chromosome. If an individual inherits the Afanasievo population with a haplogroup R1b-M269, the other individuals are from the haplogroup Q or N thus indicating a north-eastern influence. Their mitochondrial haplogroups are shared between western lineages: H, J, T, U and eastern lineages: C, A, D. Similarly, individuals in the Chemurchek culture have Oriental Y chromosome haplogroups: C and mitochondrial haplogroups Both western and eastern. The results of autosomal STR markers show that Okunevo individuals are of mixed western and eastern origin. At the end of the Bronze Age, the western haplogroup of the Y chromosome appears: R1a. It is associated with oriental haplogroups: Q, N and C. Mitochondrial haplogroups are also shared between western and eastern lineages. The results of autosomal STR markers show that these individuals are of mixed western and eastern origin. The figure below summarizes the origin of the populations tested for the three phases of the Bronze Age:  |
|
|
|
Post by Admin on Apr 6, 2017 20:04:25 GMT
 Comparing the sex-specifically inherited X chromosome to the autosomes in ancient genetic samples, we (1) studied sex-specific admixture for two prehistoric migrations. For each migration, we used several admixture estimation procedures—including ADMIXTUREmodel-based clustering (2)—comparing X-chromosomal and autosomal ancestry in contemporaneous Central Europeans, and interpreting greater admixture from the migrating population on the autosomes as male-biased migration.  For migration into late Neolithic/Bronze Age Central Europeans (“BA”) from the Pontic-Caspian steppe (“SP”), we inferred male-biased admixture at 5-14 males per migrating female. Lazaridis & Reich (3) contest this male-biased migration claim. For simulated individuals, they claim that ADMIXTURE provides biased X-chromosomal ancestry estimates. They argue that if the bias is taken into account, then X-chromosomal steppe ancestry is similar to our autosomal ancestry estimate, and that hence, steppe male and female contributions are similar. 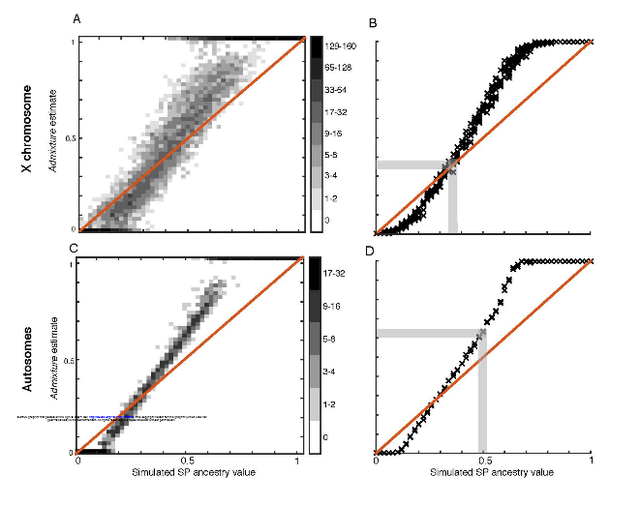 Figure 1.ADMIXTURE inference in simulated ancient genomes. Many factors affect ancestry inferences from ADMIXTURE and related programs (2,4-8). To understand A DMIXTURE inferences for X-chromosomal ancient DNA, we performed simulations examining the effects of multiple variables. First, we used “reference” individuals in (1) to simulate analogs of the BA population. Figure 1 plots estimated X-chromosomal ancestry for simulated BA individuals (Fig. 1A,B), showing that for high true ancestry levels, ADMIXTURE overestimates steppe ancestry, whereas for low levels, it underestimates it. For the intermediate ancestry in (1) (0.366), however, ADMIXTURE is accurate, and our estimate is robust to bias. As our interest in (1) was the X/autosomal comparison, we next simulated autosomes, finding bias similar to the X chromosome (Fig. 1C,D). Bias-corrected X/autosomal ancestry estimates translate in a constant-admixture model (1) to 4-7 migrating steppe males per female. Thus, accounting for ADMIXTURE bias, substantial male excess during the steppe migration remains supported. 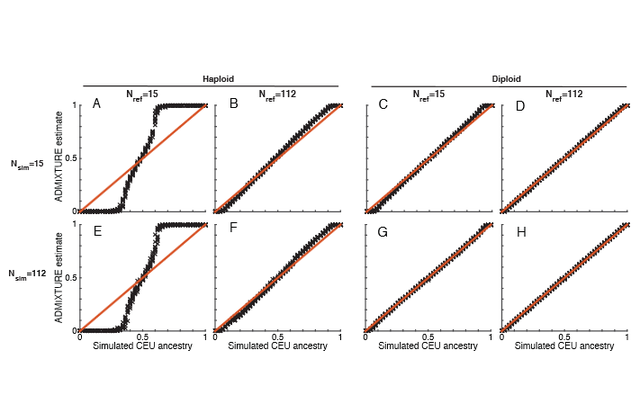 Figure 2. ADMIXTURE sample-size effects. We next tested if specific data features—haploid ancient genotypes, high missing-data rates, and small reference samples—might underlie previously unseen ADMIXTURE biases. We performed analogous simulations using modern HapMap samples without these features. This analysis traces the bias to the small reference samples available in haploid ancient data (1) (Fig. 2). We conclude that our inference of male-biased Pontic-Caspian steppe migration, seen using ADMIXTURE, STRUCTURE, mechanistic simulations, and X/autosomal FST, is robust. Our analysis further illuminates the impact of small haploid reference samples on ADMIXTURE; we look forward to refining sex-specific migration estimates as larger, higher-coverage ancient samples become available. |
|
|
|
Post by Admin on May 1, 2017 19:19:35 GMT
 Figure 1 Geographic Location and Chronologies for Latvian and Ukrainian Sites In Europe, the Neolithic transition marked the beginning of a period of innovations that saw communities shift from a mobile lifestyle, dependent on hunting and gathering for survival, to a more sedentary way of life based on food production. This new lifeway, which originated in the Near East ∼11,500 calibrated years before present (cal BP) [5, 6], had arrived in southeast Europe by ∼8,500 cal BP [7], from where it spread quickly across the continental interior of Europe and introduced animal husbandry, cultivated cereals, pottery, and ground stone tools to the region. There is a long-standing debate among archaeologists whether this spread was due to the dispersal of farmers into new lands (i.e., demic diffusion) or horizontal cultural transmission [8]. Genetic evidence suggests that these cultural and technological changes were accompanied by profound genomic transformation, consistent with the migration of people of most likely Anatolian origin [9, 10, 11, 12]. In contrast to central Europe, the adoption of agriculture in northern and eastern parts of this continent, in the areas which encompass modern-day Latvia and Ukraine, was slow and relatively recent [13, 14, 15, 16]. Although some features of the Neolithic package, such as ceramics, appeared as early as 8,500–7,500 cal BP [17, 18], agriculture was not adopted as a primary subsistence economy until the Late Neolithic/Bronze Age [13, 14, 15, 16, 19]. The Neolithic transition in the Baltic and Ukraine thus had a different tempo to that of central Europe, and it is unclear how this may have shaped the genetic composition of these regions. To investigate this, we sampled three Mesolithic and three Neolithic individuals from the archaeological site of Zvejnieki (Latvia), which is one of the richest Stone Age cemeteries in Northern Europe for number of inhumations, as well as duration of use [20, 21] (see the Supplemental Experimental Procedures for site details). We also sampled a Mesolithic and a Neolithic individual from cemeteries found along the Dnieper River in Ukraine (Vasilyevka 3 and Vovnigi 2, respectively). DNA was extracted from the petrous portion of the temporal bone (see the Experimental Procedures), which yielded between 4.30% and 55.99% endogenous DNA for all samples. Samples were shotgun sequenced using Illumina sequencing technology to between 0.22- and 4.37-fold coverage (Figure 1). The authenticity of the data was assessed in silico by examining the data for signatures of post-mortem DNA damage and evaluating the mitochondrial contamination rate in all samples along with the X chromosome contamination rate in males (see the Supplemental Experimental Procedures). All samples had degradation patterns typical of ancient DNA (Figure S1) and low contamination estimates of ∼1% or less (Table 1).  Figure 2 PCA and ADMIXTURE Analysis for Ancient Latvian and Ukrainian Samples The two earliest samples in our Baltic time series, Latvia_HG1 (8,417–8,199 cal BP), associated with the Kunda culture, and Latvia_HG2 (7,791–7,586 cal BP), associated with the Narva culture, derive from the Late Mesolithic period [17, 21]. A third sample, Latvia_HG3 (7,252–6,802 cal BP), dates to the Late Mesolithic/Early Neolithic period, with the burial showing no major departures from the preceding Mesolithic traditions [21]. Principal component analysis (PCA) with ancient samples projected onto modern Eurasian genetic variation (see the Supplemental Experimental Procedures) shows that these three hunter-gatherer samples group together in a PCA plot (first two components, Figures 2A and S1A). In keeping with their geographical origins, they are in an intermediate position between Western European hunter-gatherer samples (WHG; from Luxembourg, Hungary, Italy, France, and Switzerland) and Eastern European hunter-gatherer samples (EHG; from Russia). They are composed of the same (blue) major component as these other hunter-gatherer groups in an ancestry coefficient decomposition analysis performed using ADMIXTURE [25] (Figure 2B), suggesting a close relationship between these groups. We found that although the Latvian Mesolithic samples share closer affinity to WHG than to EHG, the Latvian Mesolithic samples do not belong entirely to either hunter-gatherer group (tested using D statistics [27], which offer a formal test of admixture; Table 2). This suggests that they may be a previously unsampled component of a hunter-gatherer meta-population that stretched across Northern Europe during the early Holocene. Next we sampled two Middle Neolithic individuals, Latvia_MN1 (6,201–5,926 cal BP), from an isolated grave located among burials from earlier periods, and Latvia_MN2 (6,179–5,750 cal BP), who was interred in a collective burial with five other individuals. During the Middle Neolithic at Zvejnieki, mortuary practices from the preceding periods were partially maintained, but some new features appeared, including collective burials and votive deposits, which are associated with the Comb Ware culture or its influences in the Baltic [21]. Despite having been roughly contemporaneous, these Middle Neolithic samples cluster in different regions of our PCA plot (Figure 2A) and have distinct profiles in ADMIXTURE analysis (Figure 2B). In both analyses, Latvia_MN1 groups with the Mesolithic Latvian samples, suggesting a degree of continuity across the Mesolithic-Neolithic transition in this region and consistent with suggestions that the eastern Baltic was a genetic refugium for hunter-gatherer populations during the Neolithic period [28]. The persistence of hunter-gatherer ancestry in the Baltic until at least the Middle Neolithic also provides a possible source for the resurgence of hunter-gatherer ancestry that is proposed to have occurred in central Europe from 7,000–5,000 cal BP [1]. In contrast, Latvia_MN2 is placed toward EHG in PCA space and has several components in ADMIXTURE analysis that are found in Native Americans, Siberians, and hunter-gatherer samples from the Caucasus. In keeping with these results, we found that there has been a northern Eurasian influence in the Baltic region since the Mesolithic period, as suggested by significantly positive statistics for the test D(Mbuti, X; Latvia Mesolithic, Latvia_MN2) when X was an EHG, modern and ancient Siberian (including the Upper Palaeolithic Mal’ta genome [29]), or Native American (Table 2). This influence is supported archaeologically by the appearance of copper rings and amber jewelry in Middle Neolithic collective burials that bear similarities to artifacts found in Estonia, Finland, and northwestern Russia [21, 30].  The latest Neolithic sample in our Baltic time series, Latvia_LN1 (5,039–4,626 cal BP), which was found in a crouched burial of the type associated with the Late Neolithic Corded Ware culture [21], falls near other Late Neolithic and Bronze Age European and Steppe samples in PCA analysis (Figure 2A). In ADMIXTURE analysis, it is composed of the blue component (Figure 2B), which is predominant in all of the older Latvian samples, but also a green component, which is maximized in hunter-gatherer samples from the Caucasus. A Caucasus-related influence in this sample is also suggested by positive results (although without formal significance, Z > 2) for tests of the form D(Mbuti, Caucasus hunter-gatherer; Latvian Mesolithic, Latvia_LN1). Ancestry related to hunter-gatherers from the Caucasus has previously been postulated to have arrived in Europe through herders from the Pontic Steppe [1, 31], and these migrations could potentially be the source of this ancestry in our sample. Interestingly, this individual lived around the time of later date estimates (∼4,500–7,000 cal BP) proposed for the split of Proto-Balto-Slavic from other Indo-European languages [3, 4]. There are two major theories to explain the distribution of Indo-European languages that constitute the most widely spoken language family in the world: (1) they have an Anatolian origin and were spread by Neolithic agriculturalists [32, 33] and (2) they developed in the Pontic Steppe and proliferated through Late Neolithic/Bronze Age migrations [1, 3, 34]. The presence of a Steppe-related component in Latvia_LN1 in the absence of an Anatolian farmer-related genetic input supports a Steppe rather than an Anatolian origin for the Balto-Slavic branch of the Indo-European language family. It is striking that we did not find evidence for early European or Anatolian farmer admixture in any of our Latvian Neolithic samples using both D statistics (Table 2) and ADMIXTURE (Figure 2A). This lack of admixture is also supported by the mitochondrial haplogroup of the Latvian Neolithic samples (all belong to U; Figure 1), which is prevalent in European hunter-gatherers [1, 35], including our Latvian Mesolithic samples, but not in early farmers. It is interesting that among the grave goods found in the burial of Latvia_LN1 was a chisel made from the bone of a domesticated goat or sheep [17, 21]. The presence of this tool made from a domesticate as well as dietary isotope data (δ15N and δ13C), which show greater reliance on terrestrial resources than in previous periods [17], is consistent with either the adoption of farming without early European farmer-related genetic admixture or the existence of trade networks with farming communities that were largely independent of genomic exchange. Although we find no genetic input from Anatolian or early European farmers in our time series, ADMIXTURE analysis of an Estonian Corded Ware sample [26] (Figure 2B) has suggested that this farmer genetic influence, which is present in contemporary Northern European populations (Figure S2), had arrived in the Baltic by at least the Bronze Age. |
|
|
|
Post by Admin on May 2, 2017 20:00:31 GMT
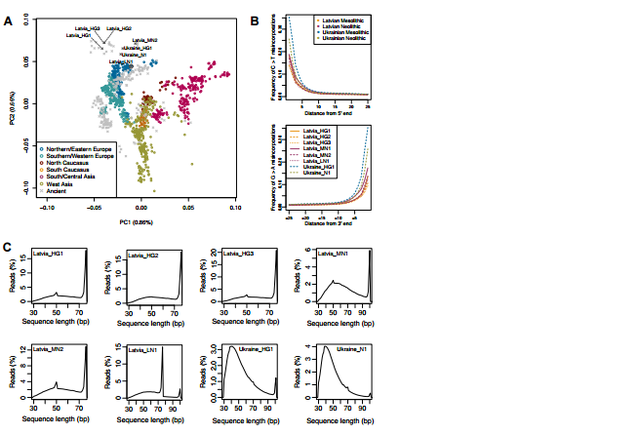 Figure S1. PCA and patterns of molecular damage. Related to Figure 2. A. PCA with modern individuals shown in colour and ancient individuals shown in grey. Datapoints are the same as in Figure 2A. B. Damage patterns for ancient samples. Plots show mismatch frequency relative to the reference genome as a function of read position for Latvian and Ukrainian samples. C. Sequence length distribution plots for ancient samples. Some samples have blips in their distributions as they were sequenced with more than one cycling format. The Ukrainian Mesolithic and Neolithic male samples (Ukraine_HG1 [11,143–10,591 cal BP] and Ukraine_N1 [6,469–6,293 cal BP], respectively) cluster tightly together between WHG and EHG samples in PCA analysis (Figure 2A). They form a clade with respect to other modern and ancient samples when tested using genome-wide D statistics (D(Mbuti, X; Ukraine_HG1, Ukraine_N1); Table S1), and their mitochondria belong to the U haplogroup, which has been found in ∼80% of European hunter-gatherer samples [1, 35]. These results suggest a degree of continuity across 4,000 years from the Mesolithic to the Neolithic period in the Dnieper Rapids. In ADMIXTURE analysis (Figure 2B), both Ukrainian samples are composed almost entirely of the European hunter-gatherer (blue) component, with a smaller green component that is also found in EHG. This green component is slightly larger in the Neolithic sample than in the Mesolithic sample, which is in keeping with D statistics that suggest increased affinity with ancient northern Eurasians from the Mesolithic to the Neolithic in Ukraine (Table S1). It is intriguing that we find an increased affinity to northern Eurasian samples in both Ukraine and Latvia during the Neolithic period. This could be the result of increased connectivity in Europe at this time. More extensive sampling will reveal whether this is a feature of the Neolithic across Northern and Eastern Europe. Relationship of Ancient Samples to Modern Populations The ancient Latvian and Ukrainian samples fall close to modern Northern and Eastern European populations in PCA analysis (Figures 2A and S1A), suggesting a degree of continuity in both regions since the Mesolithic period. Outgroup f3 statistics, which measure shared genetic drift between populations, further support this as they show that these ancient samples share most affinity with modern populations from Northern and Eastern Europe (Figure S3). Further, the Y chromosomes of two of our Latvian Mesolithic samples were assigned to haplogroup R1b (the maximum-likelihood sub-haplogroup is R1b1b), which is the most common haplogroup found in modern Western Europeans [36]. This haplogroup has been found at low frequencies before the Late Neolithic in Western Europe [1, 35] but at higher frequencies in Russia and is suggested to have spread into Europe from the East after 5,000 cal BP [1]. The presence of this haplogroup in Mesolithic Latvia points to a more westward ancestral range. We found that the three Mesolithic Latvian samples are predicted to have had the derived variant (rs12913832) of the HERC2 gene associated with blue eye color (Figure S3B and Table S2). Blue eye color is found at high frequencies in Northern Europe today, and these results suggest that this phenotype was already present in the Baltic by the Mesolithic period. We also found tentative evidence for progressive skin depigmentation in Latvia based on mutations in the SLC24A5 and SLC45A2 genes (rs1426654 and rs16891982, respectively; Figure S3B and Table S2).  Figure S3. Cross-validation error for ADMIXTURE analysis, imputed genotypes and outgroup f3-statistics. Related to Figure 2. A. ADMIXTURE analysis cross validation error as a function of the number of clusters (K). 10 replicates were performed for each value of K. The minimal error was found at K=17, but the error already started plateauing from roughly K=10, implying little improvement from this value onwards. B. Selected imputed genotypes (probability ≥ 0.7) for ancient Latvian and Ukrainian samples along with predicted hair and eye colour. The derived alleles in the SLC24A5 and SLC45A2 genes are associated with skin de-pigmenation [S1,S2] and HERC2 with light iris colour [S3,S4]. The derived allele of the LCT gene is associated with the ability to digest lactose into adulthood [S5]. Discussion The Neolithic transitions in the Baltic and Dnieper Rapids region of Ukraine show very different archaeological and genetic dynamics to those observed in Central and Western Europe. Although in central Europe pottery and agriculture arrive as a package, in the Baltic and Dnieper Rapids the onset of the Neolithic is characterized by the appearance of ceramics, with a definitive shift to an agro-pastoralist economy only occurring during the Late Neolithic/Bronze Age [13, 14, 15, 16, 19]. Although the prolonged and piecemeal uptake of Neolithic characteristics in these regions makes it challenging to attribute a definitive shift in ideology or lifestyle, it does, along with evidence for continuities in material culture and settlement patterns, suggest that Neolithic features were predominantly adopted by indigenous hunter-gatherers in this region [13, 14, 15, 16, 37]. We find genetic evidence in support of this in the affinity of the Latvian and Ukrainian Neolithic samples, Latvian_MN1 and Ukrainian_N1, to earlier Mesolithic samples from the same respective regions. However, we also find indications of genetic impact from exogenous populations during the Neolithic, most likely from northern Eurasia and the Pontic Steppe. These influences are distinct from the Anatolian-farmer-related gene flow found in central Europe during this period. It is interesting to note that even in outlying areas of Europe, such as Sweden and Ireland [38, 39], an Anatolian-farmer-related genetic signature is present by the Middle to Late Neolithic period (∼5,300–4,700 cal BP). We conclude that the gradual appearance of features associated with the Neolithic package in the Baltic and Dnieper Rapids was not tied to the same major genetic changes as in other regions of Europe. The emergence of Neolithic features in the absence of immigration by Anatolian farmers highlights the roles of horizontal cultural transmission and potentially independent innovation during the Neolithic transition. Current Biology, Volume 27, Issue 4, p576–582, 20 February 2017 |
|















