|
|
Post by Admin on Oct 2, 2019 18:21:01 GMT
Cold-climate indicators and implications for cave use We record platy microstructures in thin section for layers 12.2 (70 ± 8 to 58 ± 6 ka) and 11.4/11.2 (44 ± 5 to 38 ± 3 ka) in DCM, and for layers 13 (156 ± 15 to 146 ± 11 ka) and 11.1/9.1 (49 ± 8 to after 38 ± 9 ka) in DCE. These features, together with the presence of rounded grains and granostriated b-fabrics, which are indicative of grain rotation, indicate incipient cryoturbation. This modification of the sediment structure is most likely associated with seasonal frost, with the thinner bands in layer 9.1 of DCE possibly associated with repeated ice lensing as a result of soil creep during thaw57. The limestone clasts in these parts of the stratigraphy are generally angular and fresh, and lack signs of phosphatisation that would reflect diagenetic transformations of calcite. We therefore correlate these platy structures with the occurrence of low temperatures in the cave and relatively few freeze-thaw cycles58. In DCM, these platy microstructures are associated with sediments that contain unequivocal signs of hominin occupation (charcoal and closely associated bone fragments; Fig. 6). We do not know the vertical extent of these post-depositional features, however, so it is not clear how these signatures correlate. They may penetrate down into the underlying, older layers, but the sediments immediately above and adjacent to these samples are not affected in this way. Given the slow rate of sedimentation in the cave, we cannot rule out later over-printing of the sediments by these cold-climate indicators. 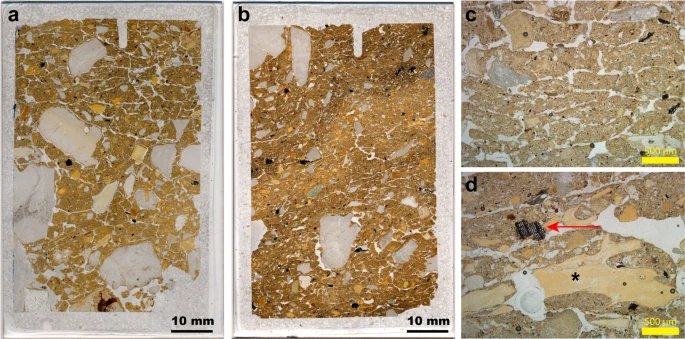 Figure 6 At the present day, thin (mm-thick) vertical cracks filled with ice have sometimes been observed within the Holocene deposits in the South Chamber. These would not, however, account for the horizontal ice lensing observed in our thin sections of layers 11.2 and 11.4 in DCM, and we see no such analogous vertical fissures in any of our thin sections. It is not clear why such platy structures and signs of incipient cryoturbation are not more common in the Pleistocene sequences at Denisova Cave, but this may relate to the enclosed cave environment mitigating extremes in temperature through restricted airflow. The South Chamber is better ventilated than are DCM and DCE, which may explain their modern occurrence there. Discussion Micromorphological analysis of the Denisova Cave sequence has provided micro-contextualised insights into the use of the site by hominins and other animals. These new data largely support previous interpretations based on field observations and other proxy datasets (e.g., faunal and pollen records6,7,8,9,35,59), thus increasing confidence in environmental reconstructions for the cave and surrounding region. Microscopic evidence for hominin use of the cave is minimal. Decades of excavation have generated significant numbers of stone artefacts6,9 that accumulated over a substantial time interval and represent multiple occupational pulses. Micro-remains of a hominin presence—such as combustion bi-products—are readily mobilised and re-deposited, so the lack of intact features indicating fire-use in the Pleistocene sequence is intriguing. Given the limited spatial area that our micromorphological study encompasses, this outcome could be due to sampling bias (e.g., ref.60). On the other hand, because easily dispersed combustion bi-products are, nonetheless, very rare, the early occupants of the site may not have been prolific pyrotechnologists. Where we do record charcoal, it is usually well preserved, so we can rule out the possibility of complete degradation of this material and its preferential removal from the sedimentary record. The abundant coprolite record shows that the cave was occupied by animals near-continuously. Cave hyena (Crocuta crocuta speleans)—the dominant carnivore in the Altai during the Pleistocene6,59—was present throughout the period of deposition of the Pleistocene sediments7,9,35. Whereas bones can accumulate at a cave site through the agency of various animals, animal droppings are most likely to be delivered directly to the cave floor. Coprolites, then, can be viewed as authigenic components of the sedimentary fill and we surmise that animals—mainly carnivores—used the sampled area of the cave throughout the time represented by the preserved sediments. Fossils of cave hyena are considerably more frequent in the DCE faunal record than are those of other Pleistocene predators, such as wolf, so hyenas are most likely the main accumulating agents of the faunal remains, given the dominance of their coprolites in the cave sediments. Coprolite fragments occur in high frequencies in layers that have been affected by frost action (e.g., layers 11.4 and 11.2 in DCE). We cannot rule out the possibility that the sampled areas fall within specific latrine areas used for ‘social defecation’61,62, perhaps exacerbated by colder temperatures driving the animals into the further recesses of the cave. At Zhoukoudian Cave in northeastern China60, rich coprolite concentrations and signs of trampling were recognised from sampling localities close to the walls of the cave, confirming the attraction of these animals to marginal zones. Profiles sampled close to the walls of caves may, therefore, fall in areas favoured for animal latrines, underscoring the importance of sampling at multiple locations throughout a site. An important outcome of our study is the identification of microstratigraphic features consistent with freezing conditions. At present, even on a sunny summer’s day, the cave interior is cold, especially so in DCE, due to the high thermal mass of the surrounding rock mitigating the warm temperatures experienced outside the cave. The cave sediments are frozen during the winter months, when temperatures can drop to an average of −16 °C in January9, but apparently not to the extent that the sediment fabric is re-arranged by ice lensing. The platy structures developed in layers deposited during late Marine Isotope Stage (MIS) 4 and MIS 3 in DCM, and during MIS 6 and MIS 3 in DCE, suggest colder conditions than those experienced in the current interglacial. We postulate that the platy features observed do not necessarily reflect the coldest conditions at the site during the Pleistocene, but are associated with specific formation environments—cold and humid conditions—that allow ice lensing to occur63. Conclusions The deposits in Denisova Cave contain microscopic traces of hominin and animal activities that illuminate the use of the cave over the last three glacial–interglacial cycles (Fig. 3). The micromorphological results show that the cave sediments are predominantly geogenic (naturally occurring), augmented by biogenic (biological) additions (e.g., coprolites, guano and digested bone) and anthropogenic inputs (e.g., charcoal, stone artefacts and associated debitage). Relationships between the various lines of evidence (e.g., micro-charcoal, bioturbation, coprolites and diagenesis), examined at a finely resolved spatial scale, reveal that hominin activities in the microstratigraphic record are few. On the other hand, coprolitic evidence for cave-dwelling carnivores is ubiquitous and suggests that the site often served as a den for hyenas and, to a lesser extent, for wolves. The cave was visited sporadically by hominins, who appeared not to have been prolific users of fire, at least in the Middle Palaeolithic deposits that constitute the majority of the Pleistocene sequence. The low frequency of hominin occupation has implications for determining the potential agency by which the few Denisovan and Neanderthal fossils were introduced to the site and their post-depositional stratigraphic integrity9,12. The environmental conditions that best preserve organic molecules, such as DNA and lipids, and whether these materials can be recovered from specific components of the microstratigraphy (e.g., coprolite fragments), also warrant further investigation. Ongoing work at Denisova Cave aims to more fully integrate micromorphology and sedimentary aDNA analyses to develop a predictive tool for organic material preservation in the deposits at this unique hominin locality. Scientific Reports volume 9, Article number: 13785 (2019) |
|
|
|
Post by Admin on Jan 11, 2020 19:13:39 GMT
INTRODUCTION In 2010, a small fragment of a finger phalanx recovered from the Denisova Cave (Denisova 3) in southern Siberia yielded a mitochondrial and a draft genomic sequence that changed our view of the evolution of the Late Pleistocene hominin lineages in Eurasia (1, 2), revealing a previously unknown archaic human population. The phylogenetic analysis of the Denisova 3 mitogenome yielded a divergence date from the ancestors of Homo sapiens and Neanderthals of around 1 million years (Ma) ago (1.3 to 0.7 Ma ago) (1, 3, 4), i.e., much earlier than the mitogenomes of the Neanderthals from the Late Pleistocene that diverged about 500 thousand years (ka) ago [690 to 350 ka ago; (3)] (Fig. 1). The nuclear genome, however, suggests a much more recent common ancestor between European Neanderthals (Vindija) and Denisovans dating to around 400 ka ago [440 to 390 ka ago; (5)], characterizing Denisovans as a sister group to Neanderthals (3, 5–8) (Fig. 1). Later, traces of an even more archaic human have been identified in the Denisova 3 nuclear genome (7), and a mitochondrial sequence related to that of Denisova 3 has been found in a ca. 400,000-year-old specimen from Sima de los Huesos (Spain), the nuclear genome of which is more closely related to Neanderthals than to Denisovans (3, 9). Together, these data suggest that the Denisovan mitogenome was either replaced with that of a more archaic human following an admixture event or represents the mitogenome of the common ancestors of Neanderthals and Denisovans before its replacement in the lineage of the Late Pleistocene Neanderthals (Fig. 1) (2–4, 9–10). The mitogenome of the late Neanderthals either could result from an introgression (i.e., replacement of the mitogenome following admixture) from early anatomically modern humans (AMHs) early after the separation of the AMH and Neanderthal populations, as proposed in one study (4), or could be due to incomplete lineage sorting given the uncertainties in the methods to estimate the dates and the wide confidence intervals of the dates proposed (Fig. 1). Furthermore, the comparison of the Denisova 3 nuclear genome with the genomic sequence of a roughly 100,000-year-old Neanderthal from the Denisova Cave revealed that Denisovans had also experienced gene flow from a Neanderthal population (Fig. 1) (7). Recently, a bone fragment, also from the Denisova Cave, has been found, through genomic analysis, to belong to a female individual that was the F1 hybrid of a Neanderthal mother and a Denisovan father (11). Her maternal Neanderthal contribution is more closely related to the genome of the 40,000-year-old European Neanderthal from Vindija (5) than to that of the ~100,000-year-old Neanderthal from the Denisova Cave. Furthermore, the paternal Denisovan genome of the hybrid appears to bear traces of an ancient Neanderthal admixture (11). These data indicate that gene flow between Neanderthals and Denisovans was not a rare occurrence.  Fig. 1 Model of the phylogeny of Neanderthal, Denisovan, and AMH populations over the past 1,400,000 years as deduced from both nuclear (blue envelope) and mitochondrial genomes (red lines). The vertical axis represents time in thousands of years (ka) ago. Population divergence dates estimated from genomic data and mitochondrial genome bifurcation date estimations originate from Prüfer et al. and Meyer et al., respectively (3, 5). Markers on the left indicate the means of the estimates for dates, and error bars indicate 95% confidence intervals. Gene flow events inferred from genome sequences are represented as dotted blue arrows (see text). Molecular dating methods based on mitochondrial sequences indicate that Denisovans must have inhabited the Altai region for over tens of thousands of years (12, 13). Despite the fact that all Denisovan mitochondrial sequences come from the same archeological site, Denisova Cave, the mitochondrial diversity of Denisovans is higher than that of Neanderthals spanning from Spain to the Caucasus (12). As inferred from the high-coverage Denisova 3 genome, the Altai population of Denisovans is characterized by low nuclear genome diversity, consistent with a prolonged small population size (10). Neanderthal populations also appeared to have been small, as assessed through the analysis of both the Altai and the Vindija genomes (5, 7). On the basis of the modeling, it has been proposed that despite reduced nuclear diversity of the individual local populations, the overall nuclear diversity of the Neanderthal metapopulation was higher (8), although this point remains under discussion as it varies with the modeling methods (6, 14). The extent of the Denisovan metapopulation diversity is still awaiting genomic characterization of remains originating from beyond the Denisova Cave, but the presence of Denisovan ancestry in modern human genomes suggests that there were at least two distinct Denisovan populations (15). Indeed, the comparison of the genomic sequence of Denisova 3 with the genomes of present-day humans has revealed interbreeding between Denisovans and early AMHs ancestral to present-day human populations not only in Southeast Asia, above all in Melanesians, but also in mainland East Asia (e.g., 15–18). The Denisovan ancestry in Melanesians appears to originate from a Denisovan population distantly related to that of the Denisova 3 specimen, and a similar ancestry can also be found in East Asia, particularly in Chinese and Japanese (15). In East Asians, a second Denisovan introgression from a Denisovan population more closely related to the Denisova 3 specimen was also detected (15). In some cases, the introgression proved to be adaptive, for example, in Tibetans (19) and Inuits (20). The distribution and diversity of Denisovan DNA in present-day human populations suggest that Denisovans were once widely distributed throughout Asia (15, 18). This evidence stands in contrast to the scarcity of unambiguously identified remains and of associated characteristic morphological features. What little morphological information that is available comes from a mandible from Xiahe on the Tibetan Plateau and three teeth from the Denisova Cave (2, 12, 13, 21). Denisovan mitochondrial genome sequences and low amounts of nuclear DNA have been recovered from a deciduous molar (Denisova 2) and two large-sized permanent molars (Denisova 4 and 8) (1, 12, 13), while the mandible has been identified as Denisovan based on proteomic information (21). The morphology of the Xiahe mandible is similar to that of the Middle Pleistocene specimens, such as the Chinese Lantian and Zhoukoudian, with features of the dental arcade shape that separate it from Homo erectus (21). It harbors some traits reminiscent of Neanderthals, while other Neanderthal-specific features are lacking (21). Thus, the rare Denisovan human remains identified to date show affinity to Middle Pleistocene hominins (2, 12, 13), particularly to those from China (21) and, to a lesser extent, to the Neanderthal lineage (12). The permanent molars from the Denisova Cave show complex occlusal morphology (1, 12, 13). Whether these peculiar characteristics of the molars are the consequence of introgression from a more archaic Eurasian population remains to be seen but cannot be excluded since such a low-level introgression has been identified in the Denisova 3 genome (7). Despite the importance of the Denisovan population for the study of human evolution, identification of Denisovan postcranial remains relies presently only on genomic data, since these remains of Denisovans exhibiting diagnostic features have yet to be reported. Progress in the identification of Denisovan skeletal remains would be instrumental for our understanding of this human lineage, for the identification of Denisovan remains, and for our ability to better characterize Denisovan population genomic diversity. Here, we report the morphometric analysis of a phalanx fragment that we show through its mitochondrial sequence to be the larger distal part of the original Denisova 3 phalanx, the genome of which had been published in 2010 and 2012 (1, 2, 10). In 2009, the phalanx was cut into two parts. The pictures of the phalanx taken by the Russian scientific team prior to its cutting, however, have been lost. The smaller proximal part of the bone was sent to the Max Planck Institute (MPI) for Evolutionary Anthropology in Leipzig, Germany, and sampling for paleogenomic analysis was performed. The larger distal part was sent to the University of Berkeley, CA, USA, and, in 2010, from there to the “Institut Jacques Monod” (IJM) in Paris, France, where it was measured and photographed and analyzed genetically. It was then returned to the University of Berkeley in 2011. The present analysis of both phalanx parts represents the first morphological study of nondental remains of this mysterious population that has inhabited Asia for hundreds of thousands of years, has interbred sometimes with Neanderthals and possibly with more archaic Eurasian humans, and continues to endure in the genomes of some present-day human populations. |
|
|
|
Post by Admin on Jan 12, 2020 2:36:32 GMT
RESULTS AND DISCUSSION Complete mitogenome sequence of the Denisova 3 fifth distal manual phalanx allows unambiguous matching of the two parts of the Denisovan phalanx The Denisova 3 phalanx (2008 Д-2/ 91) was identified in 2008 in layer 11.2 of the East Gallery, square D2, of the Denisova Cave, the date of which is assumed to be more than 50 ka ago (1, 2). To unambiguously match this distal phalanx fragment to the previously described proximal fragment, we retrieved its complete mitogenome [mitochondrial DNA (mtDNA)] sequence using DNA extraction and sequence capture procedures previously described (22–24). In total, 5838 unique reads were recovered, yielding a 26.7-fold coverage of the mitochondrial genome, of which each base was covered a minimum of twice (42 bases at twofold coverage) and a maximum of 70 times. The resulting consensus was identical to the previously published sequence (1) and represents the first replication of the Denisova mtDNA sequence outside of the MPI for Evolutionary Anthropology. This analysis also identified the previously proposed variable length in vivo of the polycytosine run at rCRS (revised Cambridge Reference Sequence) position 5889 (1), here found to be between 9 and 14 residues in length. This sequence identity indicates that the two phalanx fragments belong to the same individual. The exceptional preservation of endogenous DNA in the Denisovan phalanx is evident not only in its high endogenous DNA content but also in the length of the DNA fragments recovered. While an endogenous DNA content of 70% was recovered from the proximal fragment using the more sensitive single-stranded DNA library preparation method (10, 22), shotgun sequencing of the distal fragment analyzed in this study prepared using a double-stranded library method contained 11.3% endogenous content. This is in line with the ~6-fold increase in endogenous content reported between these two methods (10, 22). Previous analyses of the distribution of the endogenous DNA fragment lengths were performed using only merged reads, which prohibits the identification of endogenous molecules longer than 134 nucleotides. The paired-end mapping strategy used in this study reveals a more complete endogenous fragment length distribution. We show all Denisova-mapping DNA fragments to have a mean distribution of 86.7 base pairs (bp), with a median of 81.3 (Fig. 2). The mean value is similar to that first reported [mean, 85.3 bp; (1)], although these two means are skewed toward larger fragments since the library construction method used for these two studies did not recover the greater part of shorter fragments found in libraries prepared with the single-stranded library method (10). The longest fragment containing diagnostic Denisovan nucleotide sites recovered in this study was 236 bp.  Fig. 2 Distribution of DNA fragment lengths mapping to the Denisova mitochondrial sequence. Fragment lengths given in 10-bp bins are shown. Median is indicated by the dotted orange line. Read pairs that did not overlap sufficiently to be merged (i.e., longer than 134 nucleotides) but that could be mapped as paired-end reads were analyzed individually, and fragments carrying Denisovan single nucleotide polymorphisms (SNPs) were kept. In contrast to the analyses of the proximal half of the phalanx, which reported low levels of modern human DNA contamination (0.35%) (1), the distal half showed the presence of a much higher modern human mitochondrial contaminant (12.1% detected by a similar method). Further investigation revealed that this contaminant could be attributed to a single haplogroup, J1b1a1. Since no member of the IJM laboratory in Paris where the genetic analysis was performed carried mitochondrial haplogroup J, we suspect this contamination to have occurred at some point during the previous handling of the sample, prior to its preparation for genetic analysis. Anatomical description When the mitogenome analysis of the proximal epiphysis from the metaphyseal surface indicated that the specimen was not a recent modern human, it was digitized through microcomputed tomography (μCT) at the Department of Human Evolution at the MPI for Evolutionary Anthropology, Leipzig (courtesy of H. Temming and J.-J. Hublin). The reconstructed image based on these scans is shown in Fig. 3. Further sampling for nuclear DNA analysis was performed on this specimen (2), and two small holes on the articular surface witness the drilling procedure. The proximal fragment of the phalanx is now composed of two pieces (the proximal epiphysis and the remains of the dorsal part of the diaphysis) (Fig. 3).  Fig. 3 Views of the Denisova 3 DP5. (A) Virtual reconstruction of the Denisova 3 DP5 in dorsal view. Three fragments of the Denisova 3 DP5 are shown. Natural color: Photograph of the distal two-thirds of the phalanx; green: semiring of the dorsal surface of the proximal extremity of the diaphysis of the reconstituted image based on the μCT scan; blue: its proximal articular surface. (B) Virtual reconstruction based on the μCT scan of the Denisova 3 DP5 in palmar view. (C) Virtual reconstruction of the proximal articular fragment in distal (top) and lateral (middle) views, and the dorsopalmar μCT section of this piece at the level of the fusion (bottom). The D-P line (D, dorsal; P, palmar) represents the location of the section on the distal view and helps to orient the bone in the lateral view. The red arrows indicate the fusing zone. (D) Additional views of the distal fragment and the virtual reconstruction of the proximal part of the Denisova phalanx. 1 and 2: Lateral views of the distal fragment; 3: distal; 4: proximal; 5 and 6: lateral views of the proximal fragment. The gray surfaces on the outer border of the phalanx correspond to the forceps with which the phalanx was held while the photo was taken. Photos of the distal part of the phalanx were taken by E.-M.G., IJM, CNRS, Université de Paris, UMR 7592, Paris, France. The renderings of the μCT scans and the virtual reconstruction were performed by B.V., Department of Anthropology, University of Toronto, Toronto, Canada. The larger distal part of the Denisova 3 phalanx was documented in Paris by measurements with a high-precision vernier caliper (Table 1) and high-resolution images under a stereomicroscope (Leica MZ FLIII, PLANAPO 0.63×) prior to sampling (Fig. 3, A, B, and D). The reconstituted image of the entire phalanx is shown in Fig. 3 (A and B) as a virtual reconstruction in the dorsal (Fig. 3A) and palmar (Fig. 3B) views of the distal and proximal part of the phalanx, combining the photographs of the distal part and the three-dimensional (3D) model of the proximal parts. Lateral views of the distal part and several additional views of the proximal part are also shown (Fig. 3D). Measurements on the original and the rectified stereomicroscope images (Table 1) yielded identical values within ±0.1 mm for the distal and midshaft widths, showing that the pictures are accurate representations of the original bone and could be used for the purpose of a virtual reconstruction. In the original description by Reich et al. (2), the Denisova 3 phalanx is identified as “the proximal epiphysis of a juvenile manual phalanx, preserving the proximal articular surface and the bone surrounding it.” It was proposed that this distal phalanx belonged to an immature individual, probably of an age at death of at least 6 to 7 years but before the start of epiphyseal fusion. The phalanx was not determined with regard to side or ray. Here, we reanalyzed the μCT scans and photographs of the proximal fragments (the articular surface and the semiring representing the dorsal half of the proximal end of the diaphysis), as well as the photographs of the distal fragment in comparison with the distal phalanges (DPs) of Neanderthals and of Pleistocene and recent modern humans at various stages of development (table S1; see below). The distal border on this surface shows traces of the sawing of the phalanx into two pieces, consistent with those observed on the distal fragment and the proximal semiring fragment of the diaphysis. We draw the conclusion that the morphology of Denisova 3 is incompatible with an unfused immature distal phalanx. First, the palmar surface of the apical tuft is characterized by a well-defined ungual tuberosity, as is the proximal V-shaped ridge of the tuft for the insertion of the flexor digitorum profundus tendon sheath (Fig. 3B). Second, the proximal articular surface fragment on the palmar surface exhibits a relief in the middle part that is unlike the morphology of an unfused proximal epiphysis (Fig. 3B). Last, the μCT images confirm that the distal part on the palmar surface of the articular fragment is a piece of the diaphysis that is fusing (Fig. 3C, red arrows), not the original border of the proximal epiphysis. Both the semiannular dorsal surface of the diaphysis and the dorsal part of the proximal articular fragment present rounded borders that are consistent with the epiphysis fusing at the time of death (Fig. 3A). Since it takes between 2 and 4 months for an epiphysis to complete the process of fusion, once started, we conclude that the dimensions of the phalanx are close to its final mature state (25). In summary, the evidence from both the distal and proximal fragments indicates that the Denisova 3 DP5 (fifth distal phalanx) belonged to an adolescent. Nuclear DNA analyses show that this individual was a female, allowing us to narrow the age at death around 13.5 years based on the standards from extant humans and assuming that Denisovans had a fairly similar development. If we accept that the phalanx is close to the mature state, then it is possible to tentatively identify both digit and side for Denisova 3. Considering extant modern human diversity, the estimated maximum length of Denisova 3 falls best within the variability of the DP5s (25). In addition, the asymmetry of the ungual tuberosity and the curvature of the shaft in the dorsal view indicate that this DP5 is likely from the right side [(26); I. Crevecoeur, personal observation]. |
|
|
|
Post by Admin on Jan 12, 2020 5:58:02 GMT
Morphometric comparison We performed morphometric analyses of the DP5 of Denisova 3 based on the measurements taken on the original specimen and the virtual reconstruction for the maximum length (see Fig. 4A for a schematic representation of the various measurements considered here). Using univariate and multivariate analyses, we compared these measurements with data from published and unpublished DPs of Neanderthals, Pleistocene modern humans, and three samples of recent modern humans from France and Belgium dated from the Neolithic to the Middle Ages (table S1; see Fig. 4B for a direct side-by-side comparison of a DP5 from a Neanderthal, a Denisovan, and an AMH). The dimensions of Denisova 3 and the means and SDs of the comparative samples are given in Table 2. The comparison sample includes one AMH and one Neanderthal specimen in which the proximal epiphyses were in the process of fusing.  Fig. 4 Measurements of the Denisovan fifth finger phalanx and comparison with those of Neanderthals and AMHs. (A) Schematic representation of the various measurements of digital phalanges reported in Table 2 and here: ML, maximal length; PH, proximal height; PAH, proximal articular height; PB, proximal breadth; PAB, proximal articular breadth; MH, midshaft height; MB, midshaft breadth; DB, distal breadth; DH, distal height. (B) Comparison of the dorsal view of the DP5 of a Neanderthal (Krapina 206.12), a recent modern human, and the reconstructed Denisova 3. (C) Scaled Z-scores of the Denisova 3 dimensions (Den) compared to both Neanderthal (NEAND) and pooled modern human (MH) ranges of variation. Den_Neand, Den_RMH, Den_NDP5, and Den_MDP5 indicate the comparison of the values of Denisova’s DP5 with the mean and SD of each comparative group from Table 2: NEAND_DP and MH_DP, all distal phalanges; NDP5 and MDP5, DP5s only. Z-scores were scaled in a way that zero represents the mean of each range of variation, and +1/−1 represent the upper and lower 95% limits of each range of variation, respectively (see Materials and Methods). Therefore, when a value is lower than −1, it means that it falls outside the lower statistical limit (P = 0.05) of the range of variation of the comparative group in question. (D) Bivariate plot of the two first principal components of the PCA on size-adjusted measurements of the DPs. MH_DP, pooled sample of Pleistocene and recent modern human DPs; NEAND_DP, Neanderthal DPs. MH-DP5f, fusing DP5 from the modern human sample. (E) Correlation circle between the measurements of the distal phalanx and the two first principal components of the PCA on size-adjusted measurements of the DPs shown in (D). Photos of the distal part of the phalanx were taken by E.-M.G., IJM, CNRS, Université de Paris, UMR 7592, Paris, France. The renderings of the μCT scans and the virtual reconstruction were performed by B.V., Department of Anthropology, University of Toronto, Toronto, Canada. Photos of the Krapina 206.12 specimen from the Croatian Natural History Museum collections, Zagreb, Croatia, and from the recent human specimen from the collections of UMR 5199 PACEA, Université de Bordeaux, France, were taken by I.C., UMR 5199 PACEA, Université de Bordeaux, France. With the possible exception of the proximal breadth, all dimensions of Denisova 3 fall within the range of variation of modern human DP5s (Fig. 4C). The dimensions of the proximal extremity fall in the lower part of the modern human DP5 variation and outside that of the Neanderthals with regard to the articular surface measurements. This may be related to the state of preservation of the proximal extremity and, probably, also to its state of fusion. While the midshaft height of the diaphysis and the distal height of the apical tuft are close to the modern human mean, the remaining measurements fall into the lower range of the variation, indicating that the Denisova 3 phalanx is gracile. Nevertheless, the fact that we are dealing with an adolescent female must be taken into consideration with regard to potential size and gracility. We performed a multivariate analysis using size-adjusted dimensions to allow a comparison of the DPs based on shape rather than size. The projections along the two first principal components are given in the two following bivariate plots. The scatterplot of the individuals is illustrated in Fig. 4D, and the correlation circle for the original variables in Fig. 4E. The first two principal components represent more than 50% of the total variation. A clear distinction is visible between the Neanderthal and the modern human samples, with Denisova 3 positioned in the lower right quadrant within the modern human variation (Fig. 4D). As expressed by the correlation circle, the Denisovan DP5 differs from that of the Neanderthals in that the former combines a narrow apical tuft (distal breadth) with a thicker DP, particularly at the midshaft and proximal end (midshaft height and proximal height) (Fig. 4E). Neanderthal DPs have been usually described as notably different from modern humans because of their length and the shape and dimensions of their apical tufts [e.g., (27, 28)]. Neanderthal DPs are proportionally longer with wider extremities compared with modern humans, which gives the impression of flattening of the bone (29). This conformation of the apical tuft among Neanderthals seems to be related to functional rather than cold climate adaptations (30). These characteristics are confirmed by our analysis, but additional observations can be made regarding the DP5s. Neanderthal DP5s seem to occupy a specific position compared with the other Neanderthal DPs. This difference between the fifth and the other phalanges that is not visible in the modern human sample is due to both the specific morphology of the Neanderthal DP5 compared with the other digits, driven by the shape of the midshaft and the apical tuft, which are both narrower than the remaining digits. On the contrary, when not taking into account the size factor, AMH DP5s scatter within the variability of the other DPs. The nuclear genomes of Neanderthals and Denisovans are closer to each other than to modern humans, and it has been estimated that the population split time between Denisovans and Neanderthals is about 410 ka ago (5), whereas the population split time between these archaic humans and the ancestors of AMHs is about 580 ka ago (Fig. 1) (5, 7, 10). Despite being evolutionary sister groups, the Denisova 3 DP5 does not exhibit any of the features seen in Neanderthals. Its morphology is indistinguishable from that of modern humans and located within modern human variation, which likely represents the plesiomorphic morphology of nonpollical DPs within the genus Homo as seen in both the Olduvai Hominin OH 7 and the Dmanisi hominins (31, 32). This suggests that the Neanderthal-specific characters of the phalanx evolved after the divergence of Denisovans and Neanderthals. The only Neanderthal DP5 that falls in the middle of the modern human variation is from Moula-Guercy, one of the earliest members of the Neanderthal lineage from our sample dating to around 100 ka ago (33). This observation raises the possibility that the derived properties of the Neanderthal phalanx occurred rather late during the evolution of the Neanderthals. The similarity between the Denisovan phalanx and those of AMHs contrasts with the morphology of the molars of the Denisova individuals that are morphologically closer to more archaic humans from the Middle Pleistocene to the Late Pleistocene (2, 12, 13). CONCLUSIONS We could genetically link the distal part of a DP5 from the Denisova Cave in Siberia to the Denisova 3 phalanx fragment, whose genome identified it as a representative of a population more closely related to Neanderthals than to modern humans. Morphometric analysis based on high-resolution pictures, linear measurements, and the comparison with the DP5s of Neanderthals as well as Pleistocene and recent modern humans shows that it is within the range of variation of the dimensions of the DP5s of modern humans and distinct from that of Neanderthals. We propose that this represents the plesiomorphic morphology within the genus Homo (32), consistent with the morphology of early Homo DPs (31, 32), and that the derived morphology of the Neanderthal phalanx evolved after their split from the ancestors of the young woman from the Denisova Cave (see Fig. 1). This finding calls for caution when identifying potential Denisovan postcranial skeletal remains beyond Denisova, as their morphology might be ambiguous or more similar to modern humans than to Neanderthals. Science Advances 04 Sep 2019: Vol. 5, no. 9, eaaw3950 |
|
|
|
Post by Admin on Jan 15, 2020 2:24:06 GMT
 Now, scientists say this influence may be more expansive than they previously thought. In fact, ancient humans’ genetic exchange could be one of the major causes of adaptive evolution in humans, according to a new study. Using new computational methods, scientists determine that the gene flow between archaic humans affects modern-day human metabolism, our response to different types of pathogens, and a scattering of neuronal traits. The findings were published on Tuesday in Molecular Biology and Evolution. We realized that such interactions for Neanderthal and Denisova-inherited mutations had been overlooked.” Study authors Alexandre Gouy and Laurent Excoffier first analyzed “archaic introgression maps” for 35 Melanesian individuals. Introgression maps, Gouy tells Inverse, tell you which blocks in your genome are likely to be of archaic ancestry. They’re traced by comparing the genomes of ancient hominins — obtained from Neanderthal and Denisovan fossils — and modern humans using statistical tools. Basically, you can “see a genome as a mosaic of blocks inherited from your ancestors,” he says. As ancient hominins interbred with modern humans, some of these blocks along the genome can be traced back to Neanderthal and Denisovan ancestors. The researchers then looked at introgression maps across participants in the 1,000 Genomes Project. For the purposes of the study, the researchers focused on those of people from East Asia, Europe, and Papua New Guinea.  What did humans inherit? Their analysis of patterns of introgression, along with data sets of connected genes and subnetworks, yielded complex and fascinating findings. It’s previously been shown that the Denisovan gene EPAS1 likely helps Tibetans live in high-altitude places, and that some Neanderthal variants are associated with behavioral traits, including mood disorders and an inclination towards cigarettes. Neanderthal traits Neanderthal DNA influences particular traits in humans, previous studies established. The new study found that, in European populations, Neanderthal genes are also linked to metabolism, iron- and oxygen-binding in red blood cells and muscles, as well as olfactory receptors. Among East Asians and Europeans, ancient introgression is associated with a GABA transporter and a neurotransmitter transporter, the study suggests. In Papuans, genes showing “a significant excess of introgression” associated to autism susceptibility and attention deficit-hyperactivity disorder (ADHD) were found. Especially intriguing was the finding the presence of introgressed mutations in Papua New Guineans that are potentially linked to resilience to malaria, Guoy says. These mutations are linked to Denisovan ancestry. 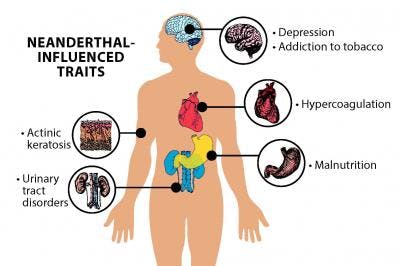 Not all inheritance is the same Importantly, just because one has Densivoan or Neanderthal DNA in their genome that doesn’t mean that inheritance is going to show up in their genes in the same way. Each human population has a specific history, and ancient hominins interbred with modern humans at different times and in different places. “That is why introgressed genes are sometimes specific to a population,” Gouy says. “Different people can carry the same amount of Neanderthal DNA, but it can be found in different places of their genome.”  For example: The region of the genome that may be involved in resistance to malaria among Papua New Guineans is inherited from Denisovans. These mutations are almost always found in Melanesians and Aboriginal Australians — which is why they aren’t present across the global population. It’s also not as simple as saying because a person with Neanderthal DNA has ADHD, then Neanderthals had ADHD. While this study points out that Denisovan and Neanderthal-inherited genes are related to health and behavior, “it remains very difficult to quantify precisely the effect of those mutations,” Guoy says. “What we can say so far is that some introgressed mutations have been associated to neurological processes,” he says. “We cannot know yet precisely how it will affect the health or behavior of an individual, based on genomic data only.”  A different way of examining gene interactions The study is based on two novel approaches, Guoy says. One allows researchers to find networks of genes showing an excess of introgression in particular populations, and the one to other tests whether specific mutations in certain genes tend to be found together in modern individuals. That clumping is known as when genes are “co-introgressed.” These techniques allowed them to gain new insights by examining the data from a network-interaction perspective. Gouy says that they can see their approach as complementing more traditional methods that focus on single genes, this simply allows them to take a different perspective on the same data. “I personally find it fascinating to see that interbreeding with other human lineages shaped human adaptations,” Gouy says. “As we were developing approaches to understanding modern human adaptations by looking at gene interactions, we realized that such interactions for Neanderthal and Denisova-inherited mutations had been overlooked.” The results from genomic studies need to be interpreted with caution, Gouy says. Behavior results from a complex interaction of genes and the environment — and it’s difficult to assess the full impact genes have. But it is obvious that the interaction between genes affects us in some way, and historically our archaic mutations have been overlooked. These played a role in human evolution and health, and more research is needed to know the full extent. Abstract:Anatomically modern humans carry many introgressed variants from other hominins in their genomes. Some of them affect their phenotype and can thus be negatively or positively selected. Several individual genes have been proposed to be the subject of adaptive introgression, but the possibility of polygenic adaptive introgression has not been extensively investigated yet. In this study, we analyze archaic introgression maps with refined functional enrichment methods to find signals of polygenic adaptation of introgressed variants. We first apply a method to detect sets of connected genes (subnetworks) within biological pathways that present higher-than-expected levels of archaic introgression. We then introduce and apply a new statistical test to distinguish between epistatic and independent selection in gene sets of present-day humans. We identify several known targets of adaptive introgression, and we show that they belong to larger networks of introgressed genes. After correction for genetic linkage, we find that signals of polygenic adaptation are mostly explained by independent and potentially sequential selection episodes. However, we also find some gene sets where introgressed variants present significant signals of epistatic selection. Our results confirm that archaic introgression has facilitated local adaption, especially in immunity related and metabolic functions and highlight its involvement in a coordinated response to pathogens out of Africa. |
|











