|
|
Post by Admin on Oct 17, 2019 20:34:46 GMT
Island Southeast Asia (ISEA) represents a key region for the study of hominin evolution and interaction. Several extinct hominin groups populated this region, and current inhabitants harbor both Neanderthal and Denisovan ancestry in their genomes. Fossil evidence indicates the presence of Homo erectus on Java from ~1.7 million years (Ma) ago to somewhere between 53 and 27 thousand years (ka) ago (1, 2), Homo floresiensis on Flores from 100 to 60 ka ago (3–5), and modern humans in Sulawesi by 40 ka ago (6). Furthermore, the highest Denisovan ancestry is found in this region, in people living east of Wallace’s Line (7), a stark faunal boundary representing the ancient and persistent deep-water separation of the Sunda and Sahul lands. Much of the genetic ancestry of modern ISEA groups derives from the Austronesian expansion, a demographic event that carried genes from mainland Asia 4 to 3 ka ago (8). However, it remains unclear how selection and admixture with archaic hominins in some remote island groups may be related to this expansion and contemporary populations in this region. The history of hominin presence on Flores Island is particularly enigmatic. Found 400 km east of Wallace’s Line, Flores is home to skeletal remains of the diminutive species H. floresiensis (estimated height of ~106 cm) (3, 4), which inhabited this biogeographical setting from ~0.7 Ma ago until 60 ka ago (5, 9). Archaeological evidence indicates hominin presence on Flores by 1 Ma ago (10). More recent remains of short-statured humans have also been found at cave sites on the island (11). Current Flores inhabitants include a pygmy population living in the village of Rampasasa (12), near the Liang Bua cave where H. floresiensis fossils were discovered (3). We collected DNA samples from 32 adult individuals from Rampasasa (average height of 145 cm) (Fig. 1A and table S1) (13) and generated genotype data for ~2.5 million single-nucleotide polymorphisms (SNPs) (fig. S1). On the basis of family relationships and ancestry inferred from SNP data (table S2 and figs. S2 and S3), we selected 10 individuals for whole-genome sequencing (median depth of 37.8x; genotype concordance >99.8%) (table S3). The sequenced individuals include a trio to facilitate haplotype inference (13), but only nine unrelated individuals were considered for downstream analyses.  Fig. 1 Sampling location and genomic variation of the Flores pygmies. (A) Location of Flores pygmy village and populations integrated into the analysis. The inset shows a subset of 85 populations from East Asia (EA), ISEA, and Oceania used for the PCA. The Rampasasa village (red square) is close to the Liang Bua cave, where H. floresiensis fossils were excavated. (B) PCA performed on 85 populations (13). (C) ADMIXTURE results for K = 6 clusters are shown for 96 worldwide populations (13). To infer population relationships, we integrated our sequencing data with SNP array data from 2507 individuals spanning 225 worldwide populations, as well as sequencing data from Melanesia (13). A principal component analysis (PCA) places the Flores population in close proximity to a cluster of East Asian and ISEA samples, with a notable affinity toward Oceanic populations (Fig. 1B and table S4). Population structure inferred by ADMIXTURE shows that most of the ancestry in the Flores pygmies can be explained by an East Asian–related component and by a smaller New Guinean–related component, shared with Oceanic populations (Fig. 1C and fig. S4). The New Guinean component accounts for 23.2% of Flores ancestry (z-score > 85) (fig. S5) (13). These results, along with multiple sequential Markovian coalescent inferences of effective population size and divergence times, analyses of inbreeding, and mitochondrial DNA and Y chromosome variation, indicate that the Flores pygmies likely trace their ancestry back to the ancestors of Near Oceanic populations and experienced a recent admixture event with populations of East Asian ancestry (figs. S6 to S11 and tables S4 and S5) (13). We used a PCA projection method to describe the relationship between the Flores pygmies and archaic hominins (13). Flores individuals exhibit affinity to both Neanderthals and Denisovans, suggesting that they harbor ancestry from both archaic hominins (Fig. 2A). Using F4 ratio statistics, we estimated that the Flores pygmies harbor, on average, 0.8% Denisovan ancestry (z-score > 4) (13), which is higher than the amount for other ISEA populations but lower than that for Oceanic populations. Consistent with previous observations (7), the amount of inferred Denisovan ancestry is positively correlated with proportion of New Guinean ancestry (Pearson’s correlation = 0.97, P < 10−16) (fig. S12) (13).  Fig. 2 Archaic hominin ancestry in the Flores pygmies. (A) PCA to investigate genetic similarities of present-day humans and archaic hominins. Mean values for the top two principal components were plotted for each population. (B) Amounts of total archaic introgressed sequences in 9 Flores pygmies, 27 Melanesians, 103 East Asians, and 91 Europeans. The inset shows amounts of Neanderthal, Denisovan, and ambiguous sequences in Flores and Melanesian individuals. (C) The Denisovan D duplication is present only in Denisovan, Oceanic, and Flores individuals. A panel of worldwide populations is shown, along with the Denisovan and Neanderthal genomes (13). A copy number greater than two (four and three for light and dark blue, respectively) in region D (far right) indicates presence of the duplication. We identified regions inherited from archaic hominins by applying the S* statistical framework (fig. S13) (13) to 9 Flores, 27 Melanesian, 103 East Asian, and 91 European genomes. On average, we retrieved 53.5 Mb of significant S* sequence in the Flores sample (false discovery rate ≤ 5%) (Fig. 2B). Of this, 47.5 and 4.2 Mb were assigned with high confidence to the Neanderthal and Denisovan groups, respectively, whereas 1.8 Mb were classified as “ambiguous” (for which Neanderthal or Denisovan status cannot be confidently distinguished) (Fig. 2B, inset, and fig. S14). The average amount of Neanderthal sequence per individual (47.5 Mb) among Flores pygmies is intermediate between that among East Asians (54.5 Mb) and Melanesians (40.2 Mb), whereas the average amount of Denisovan sequence was less than that identified in Melanesians (32 Mb) (fig. S14). These data suggest that the source of Denisovan ancestry was localized east of Wallace’s Line and that such ancestry was diluted in Flores by subsequent admixture with Asian populations carrying less (or no) Denisovan ancestry (14). The S* statistic does not require information from an archaic reference genome and thus can potentially identify sequences from unknown hominin lineages. We searched for signatures of admixture with an unknown archaic hominin source by analyzing significant S* sequences that did not match the Neanderthal or Denisovan genomes (hereafter called “unknown” sequences) (fig. S14). We found no evidence that unknown sequences in Flores are enriched for older or more divergent lineages (figs. S15 and S16) (13), as would be expected if they contained lineages inherited from a more deeply divergent hominin group, such as H. floresiensis or H. erectus. Although it is difficult to exclude very low levels of admixture from such groups given current methodological limitations, our data are inconsistent with substantial levels of ancestry from a deeply divergent hominin lineage. We analyzed copy number variation (CNV) in the Flores pygmies, along with a panel of diverse modern and archaic human genomes (figs. S17 to S24 and tables S6 and S7) (13). We found 1865 biallelic CNVs in Flores individuals, as well as a common [allele frequency (AF) = 50%], large segmental duplication block (>220 kilo–base pairs; chromosome 16p12.2) that to date has been observed only in Denisovan and Oceanic individuals (AF = 82.6%) (Fig. 2C and fig. S18). Previous work suggests this duplication introgressed from Denisovans into the ancestors of present-day populations in Oceania ~40 ka ago (15). |
|
|
|
Post by Admin on Oct 18, 2019 18:37:38 GMT
To test hypotheses of recent adaptation, we used the population branch statistic (PBS) to scan for alleles exhibiting strong population-specific structure (13). We identified 2 genomic windows in the top 0.001 percentile (PBS > 1.04) and 10 additional windows in the top 0.01 percentile (PBS > 0.78) of mean genome-wide PBS scores (fig. S25 and table S8). One of the top two regions encompasses the human leukocyte antigen gene complex, a well-known substrate of diversifying selection with a critical role in adaptive immunity (13). The strongest PBS signal, however, extends over a ~74-kb region of chromosome 11 that includes FADS1 and FADS2 (Fig. 3A). These genes encode fatty acid desaturase (FADS) enzymes that catalyze synthesis of long-chain polyunsaturated fatty acids (LC-PUFAs) from plant-based medium chain (MC)–PUFA precursors. Notably, the Flores sample is nearly fixed for an ancestral haplotype (tagged by the C allele of SNP rs174547) in a pattern consistent with a recent selective sweep. In the larger Omni2.5-genotyped sample (n = 21 unrelated individuals), we confirmed a 95% frequency of the ancestral (C) allele of rs174547. Other Southeast Asian populations also carry high frequencies of the ancestral allele (Fig. 3B, inset), consistent with positive selection in their common ancestor, with drift and additional selection in ISEA populations subsequent to their divergence (13).  Fig. 3 Population genetic and functional signatures at the FADS locus. (A) LocusZoom local Manhattan plot showing individual SNP PBS values spanning the FADS locus. Haplotype-defining SNP rs174547 is shown in dark gray, whereas other SNPs are colored according to pairwise linkage disequilibrium with rs174547 (from East Asian populations from the 1000 Genomes Project). (B) Geography of Genetic Variants map at rs174547. Data from Flores (n = 18 haplotypes), Melanesian (n = 54 haplotypes), and the Greenlandic Inuit (n = 4 haplotypes) populations are overlaid on populations from the 1000 Genomes Project. SNP array data from the Human Origin Dataset are shown in the inset. (C) Multitissue eQTL data from the GTEx (Genotype-Tissue Expression) Project, depicting associations between FADS1 and FADS2 expression and the rs174547 genotype. Effect size is displayed on the x axis and by color, whereas significance is indicated by point sizes. Supporting functional differences, previous data demonstrate that SNPs defining this haplotype are strongly associated with circulating levels of fatty acids (16) (table S9) and a wide variety of blood phenotypes (17) (table S10). Furthermore, these variants are known expression quantitative trait loci (eQTLs) of both FADS1 and FADS2 (13, 18, 19). Specifically, the selected (C) allele of rs174547 is associated with up-regulation of FADS2 and down-regulation of FADS1 (20) (Fig. 3C), in turn predicting reduced efficiency of conversion from MC- to LC-PUFA. Our data add to an emerging body of evidence suggesting that the ancestral and derived haplotypes at the FADS locus have been targeted by independent episodes of positive selection in geographically diverse populations (18, 21–23). Notably, an ancestral haplotype at FADS is nearly fixed in an Inuit population in Greenland (23), potentially in response to climate and a marine diet rich in omega-3 fatty acids. While mirroring our findings from Flores, the adaptive Greenlandic Inuit haplotype extends over a broader downstream region encompassing FADS3, potentially reflecting distinct selection events or population-specific patterns of recombination (fig. S26). Although the global history of this locus remains to be clarified, current evidence points to a critical role of FADS as an evolutionary “toggle switch” in response to changing diet. Body size reduction is one of the best-known responses to island environments and is common among mammalian taxa. Examples on Flores include H. floresiensis and the pygmy proboscidean Stegodon. We leveraged our data to test hypotheses about the genetic architecture and evolution of short stature in our Flores sample. If the short-stature phenotype on Flores was a consequence of polygenic selection acting on common variants, we would expect to see higher frequencies of alleles associated with reduced height in other populations (24). We thus performed mixed-linear model association analysis for height in 456,426 individuals of European ancestry to identify height-associated loci, unbiased of population stratification (13). We find that European height-associated loci are significantly more differentiated between Flores and other neighboring populations than expected under random genetic drift (Fig. 4A). Moreover, the Flores sample is significantly enriched for height-decreasing alleles (test of population genetic differentiation across all SNP sets P < 0.001; correlation of AF differentiation and allele effect size at 4000 alleles of strongest height association: −0.71, t = −3.18, df = 4000, P = 0.002) (Fig. 4B). This result predicts a smaller height for Flores individuals from height-associated alleles discovered in an unrelated panel (Fig. 4A), and we estimate that 36.6% (95% confidence interval: 10.4 to 63.9%) of variation in a genome-wide genetic predictor of height is attributed to mean differences among the populations. Assuming the phenotypic standard deviation (SD) of height in this population is 6 cm and the full heritability is 0.7 (25), then one genetic SD = 0.7 × 6 = 5 cm. Because the genetic predictor explains 8.5% (SE: 3.8%) of phenotypic variance in Flores (Fig. 4C) and the Flores population is 1 SD lower than the average of neighboring populations (Fig. 4A), we predict the Flores population to be ~2 cm (0.085 × 6 = 1.75 cm) shorter in stature than populations in East Asia and Oceania and ~5 cm shorter if the genetic predictor explained 70% of the phenotypic variance. Collectively, these data provide evidence that polygenic selection acting on standing genetic variation was an important determinant of short stature in this Flores pygmy population.  Fig. 4 Polygenic selection for reduced stature in the Flores pygmies. (A) Comparison of the genome-wide genetic predictor of height from observed genotypes (purple) versus the null model (blue). The Flores panel is significantly enriched for height-reducing alleles (P < 0.001) in a multivariate chi-square test of population genetic differentiation from the expectation under the null model. (B) Frequency differences between the Flores population and neighboring 1000 Genomes Project populations for 4000 genome-wide loci of the strongest association, with height plotted against effect size for the height-increasing allele. The regression slope shown in the figure between the height-increasing effect size and the frequency difference was −0.71 (t = −3.18, P = 0.002), reflecting height-increasing alleles being at lower frequency in the Flores population. (C) Association between height in the Flores population and a genetic predictor of height. High-coverage genomes provide insights into the history of demographic changes and adaptation in a pygmy population on Flores Island (Indonesia). Although these individuals possess ancestry from both Neanderthals and Denisovans, we found no evidence of admixture with a deeply diverged hominin group. This observation, combined with the evidence that their short-stature phenotype resulted from polygenic selection acting on standing variation, suggests that insular dwarfism arose independently in two separate hominin lineages on Flores Island. Science 03 Aug 2018: Vol. 361, Issue 6401, pp. 511-516 |
|
|
|
Post by Admin on Oct 30, 2019 18:31:31 GMT
Quantitative genetics of body size evolution on islands: an individual-based simulation approach Abstract According to the island rule, small-bodied vertebrates will tend to evolve larger body size on islands, whereas the opposite happens to large-bodied species. This controversial pattern has been studied at the macroecological and biogeographical scales, but new developments in quantitative evolutionary genetics now allow studying the island rule from a mechanistic perspective. Here, we develop a simulation approach based on an individual-based model to model body size change on islands as a progressive adaptation to a moving optimum, determined by density-dependent population dynamics. We applied the model to evaluate body size differentiation in the pigmy extinct hominin Homo floresiensis, showing that dwarfing may have occurred in only about 360 generations (95% CI ranging from 150 to 675 generations). This result agrees with reports suggesting rapid dwarfing of large mammals on islands, as well as with the recent discovery that small-sized hominins lived in Flores as early as 700 kyr ago. Our simulations illustrate the power of analysing ecological and evolutionary patterns from an explicit quantitative genetics perspective.  Modeling generations on the island The Hobbit’s most likely ancestor is Homo erectus, a species more than twice its size in terms of its brain and overall bulk. Based on the geological history of Flores and the oldest known fossils of Homo floresiensis, it seems the evolution of the new species must have occurred in less than about 300,000 years. As evolutionary biologists, we are acquainted with the idea that Darwinian evolution is a slow and gradual process that takes place over very long timescales. Could such drastic change in body size happen this fast? So our interdisciplinary research team developed a computer model to try to answer this basic question. It’s like a computer game that simulates body size evolution under biologically and ecologically realistic scenarios. In our model, individuals colonize the island, grow to their adult body size according to how much food is available, give birth to a number of young and die. The basic rule of the game is that individuals that are closer to the ‘optimum’ body size for the island in that moment will leave more descendants. Offspring inherit genes for large or small body size. Generation after generation, new mutations may appear in the population and shift body size toward either higher or lower values. Occasionally, new individuals might even invade the island and mix with the residents. Another basic rule is that the initial small population cannot grow above the number the island’s resources might sustain. Our colleagues, Earth systems scientists Neil Edwards and Phil Holden, used paleoclimatic data to tweak our model. Hotter and wetter times can support more people on the island, and would influence optimum body size at any given moment. We started our simulations assuming that large-bodied Homo erectus arrived at the island and then evolved into a smaller species there. Since we just don’t know the exact numbers our model should crank through, we based them on estimates obtained from current human populations. Because of this uncertainty, we ran our model thousands of times, each time using a random combination of all the parameters. Ultimately we were able to build a statistical distribution of how long it took for Homo erectus to become as small as Homo floresiensis.  A new species, in the blink of an evolutionary eye After running 10,000 simulations, we were surprised to discover that in less than 350 generations, the process was complete. Thinking in terms of years, assuming a young female delivers a first baby at the average age of 15, that translates to about 10,000 years. That may seem long for you and me. But from an evolutionary perspective, that’s the blink of an eye — a little more than a thousandth of Homo evolutionary history. Of course we do not expect that all the features that make Homo floresiensis as unique as it is evolved that fast and at the same time. Yet, our simulation still shows, 300,000 years is far more than enough time for a new human species to arise. Our work supports the idea that fast evolution is quite plausible under a realistic set of ecological parameters, and that natural selection may be a powerful force influencing body size on islands. And if Homo floresiensis is indeed a product of the island rule, she shows — yet again — that we humans tend to obey the same overall rules driving evolution in many other mammals. _____ |
|
|
|
Post by Admin on Dec 19, 2019 2:09:07 GMT
 An ancient relative of modern humans survived into comparatively recent times in South East Asia, a new study has revealed. Homo erectus evolved around two million years ago, and was the first known human species to walk fully upright. New dating evidence shows that it survived until just over 100,000 years ago on the Indonesian island of Java - long after it had vanished elsewhere.  In the 1990s, one team came up with unexpectedly young ages of between 53,000 and 27,000 years ago. This raised the distinct possibility that modern humans overlapped with Homo erectus on the Indonesian island. Now, researchers led by Prof Russell Ciochon of the University of Iowa in Iowa City opened up new excavations on the terraces beside the Solo River, reanalysing the site and its surroundings. They have provided what they describe as a definitive age for the bone bed of between 117,000 and 108,000 years old. This represents the most recent known record of Homo erectus anywhere in the world. "I don't know what you could date at the site to give you more precise dates than what we've been able to produce," Prof Ciochon told BBC News.  Prof Chris Stringer, research leader on human evolution at London's Natural History Museum, who was not involved with the work, commented: "This is a very comprehensive study of the depositional context of the famous Ngandong Homo erectus partial skulls and shin bones, and the authors build a strong case that these individuals died and were washed into the deposits of the Solo River about 112,000 years ago. "This age is very young for such primitive-looking Homo erectus fossils, and establishes that the species persisted on Java for well over one million years." Researchers think the collection of remains represent a mass death event, possibly the result of a lahar upriver. A lahar - which comes from a Javanese word - is the slurry that can flow down the slope of a volcano when heavy rainfall occurs during or after a volcanic eruption. These violent events will sweep away anything in their path.  Ciochon and his colleagues excavated part of an untouched reserve area left alone by the Dutch team in the 1930s. Informed by records of the original excavations, the team was able to identify the gravelly deposit - or bone bed - from which the Homo erectus fossils had come, and date it. On other islands in South-East Asia, Homo erectus appears to have evolved into smaller forms, such as Homo floresiensis - the "Hobbit" - on Flores, and Homo luzonensis in the Philippines. This probably occurred because there were limited food resources on these islands. But on Java, there appears to have been enough food for erectus to maintain its original body size. The specimens at Ngandong appear to be between 5ft and 6ft in height - comparable to examples from Africa and elsewhere in Eurasia. But why did Homo erectus survive so late on Java? In Africa, the species was probably gone by 500,000 years ago; in China it vanished some 400,000 years ago. Russell Ciochon thinks that it was probably outcompeted by other human species elsewhere, but Java's location allowed it to thrive in isolation. However, the results show the fossils came from a period when environmental conditions on Java were changing. What were once open woodlands were transforming into rainforest. Prof Ciochon thinks this could mark the exact point of extinction of Homo erectus on the island.  Last appearance? No Homo erectus are found after this time, he explained, and there's a gap with no human activity at all until Homo sapiens turns up on Java around 39,000 years ago. Prof Ciochon believes H. erectus was too dependent on the open savannah and too inflexible to adapt to life in a rainforest. "Homo sapiens is the only hominin species that lives in a tropical forest," he explained. "I think it's mainly because of the cultural attributes of Homo sapiens - the ability to make all these specialised tools." "Once this rainforest flora and fauna spread across Java, that's the end of erectus." 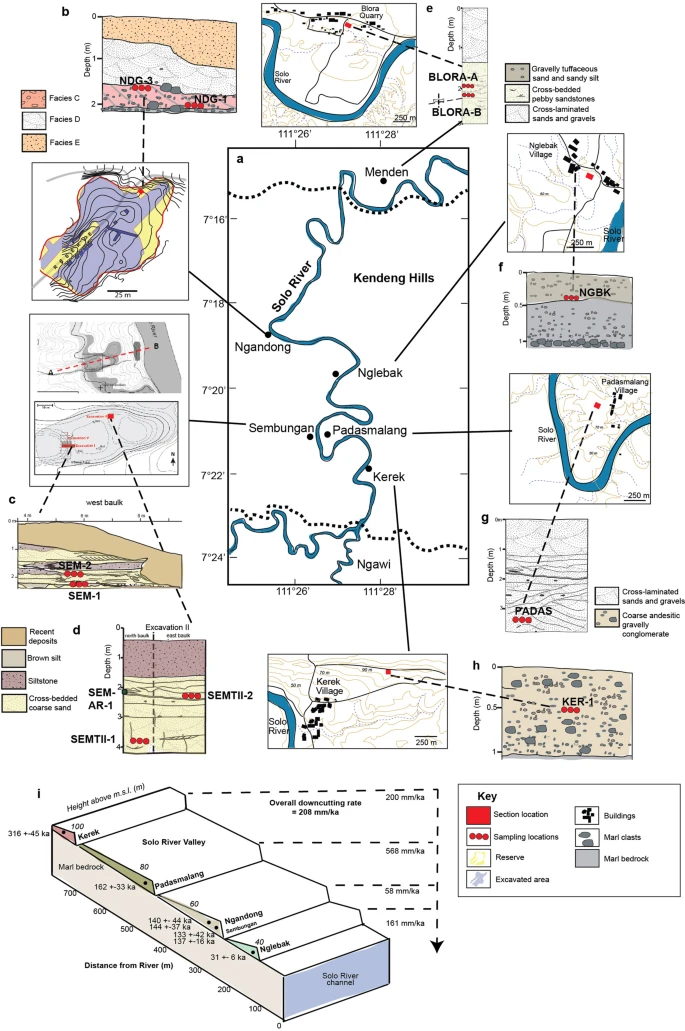 Extended Data Fig. 2 Terrace-site stratigraphy and luminescence sampling. But Chris Stringer sounded a note of caution. "The authors claim that this is therefore the last known occurrence of the species, and that this indicates there was no overlap of the species with Homo sapiens in Java, as H. sapiens arrived much later," he said. "I'm not convinced about that as other supposedly late Homo erectus material from Javanese sites like Ngawi and Sambungmacan remain to be properly dated, and they may be younger still. Alternatively, they may correlate with the ages of the Ngandong fossils, but that should be the next stage of investigation." |
|
|
|
Post by Admin on Dec 20, 2019 6:48:52 GMT
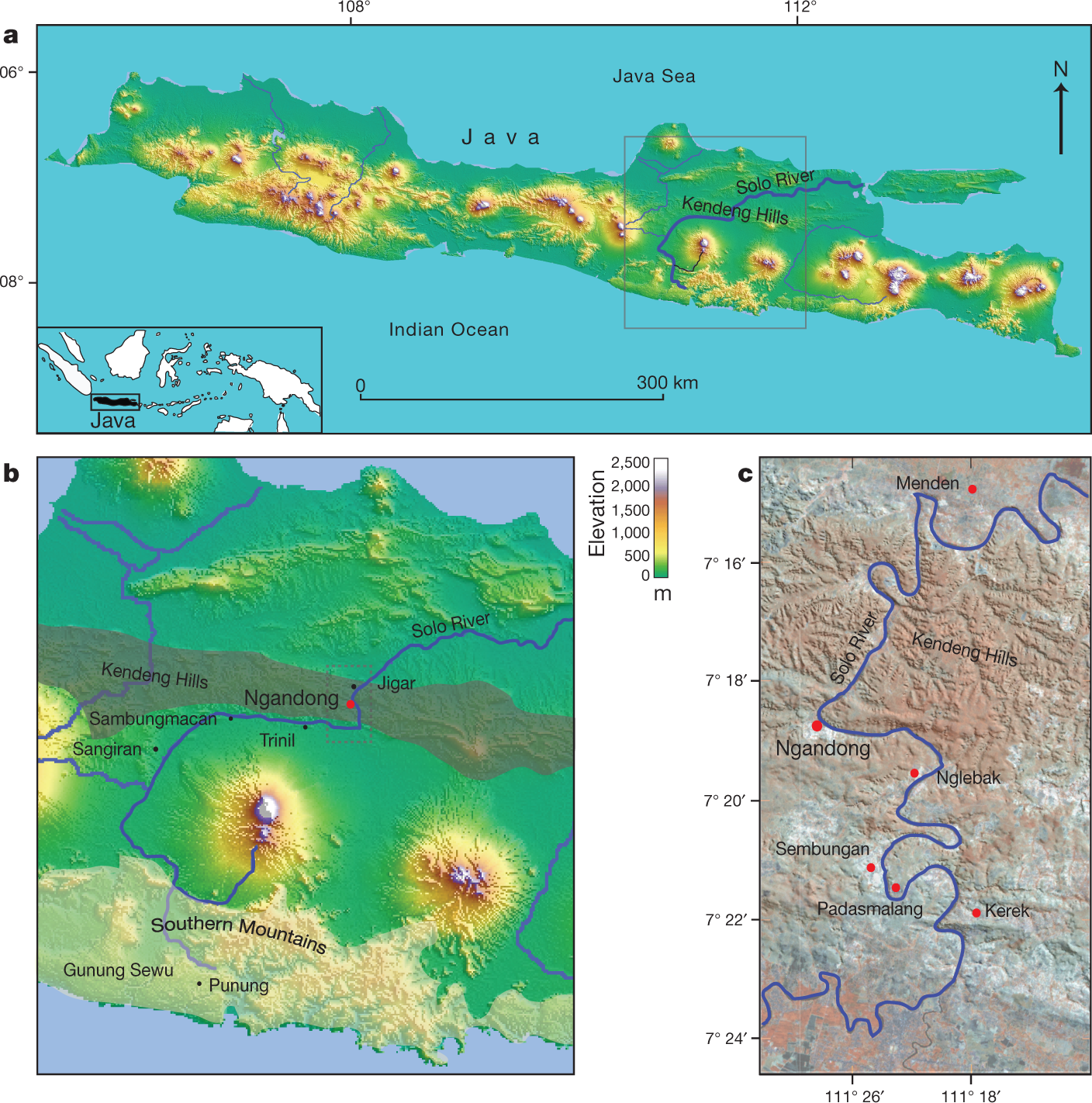 Fig. 1: Location of Java with H. erectus sites and key study sites on the terraces. Abstract Homo erectus is the founding early hominin species of Island Southeast Asia, and reached Java (Indonesia) more than 1.5 million years ago1,2. Twelve H. erectus calvaria (skull caps) and two tibiae (lower leg bones) were discovered from a bone bed located about 20 m above the Solo River at Ngandong (Central Java) between 1931 and 19333,4, and are of the youngest, most-advanced form of H. erectus5,6,7,8. Despite the importance of the Ngandong fossils, the relationship between the fossils, terrace fill and ages have been heavily debated9,10,11,12,13,14. Here, to resolve the age of the Ngandong evidence, we use Bayesian modelling of 52 radiometric age estimates to establish—to our knowledge—the first robust chronology at regional, valley and local scales. We used uranium-series dating of speleothems to constrain regional landscape evolution; luminescence, 40argon/39argon (40Ar/39Ar) and uranium-series dating to constrain the sequence of terrace evolution; and applied uranium-series and uranium series–electron-spin resonance (US–ESR) dating to non-human fossils to directly date our re-excavation of Ngandong5,15. We show that at least by 500 thousand years ago (ka) the Solo River was diverted into the Kendeng Hills, and that it formed the Solo terrace sequence between 316 and 31 ka and the Ngandong terrace between about 140 and 92 ka. Non-human fossils recovered during the re-excavation of Ngandong date to between 109 and 106 ka (uranium-series minimum)16 and 134 and 118 ka (US–ESR), with modelled ages of 117 to 108 thousand years (kyr) for the H. erectus bone bed, which accumulated during flood conditions3,17. These results negate the extreme ages that have been proposed for the site and solidify Ngandong as the last known occurrence of this long-lived species. 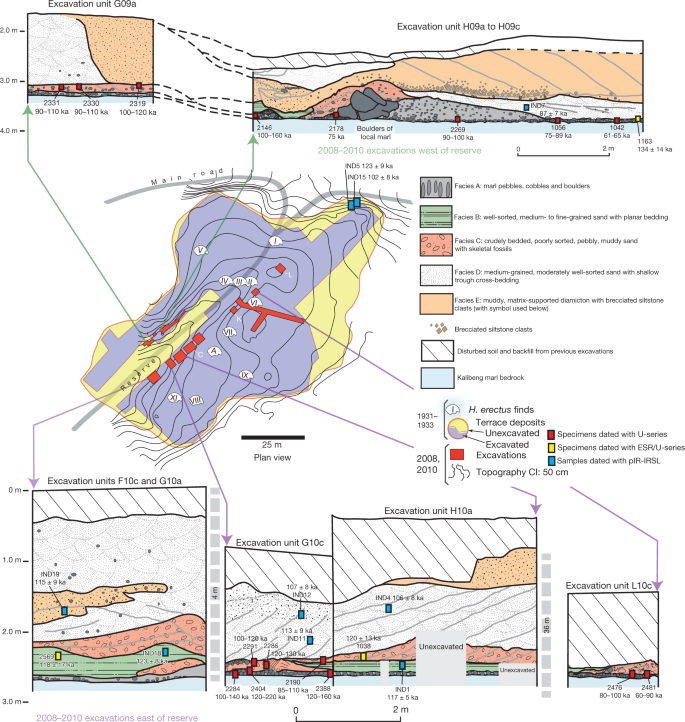 Fig. 2: Cross-sections of Ngandong site, showing the stratigraphic context and the location of our dating samples. To address these chronological inconsistencies, we applied a regional approach to establishing a chronology for the re-excavations that we conducted at Ngandong (Fig. 2). First, to establish a landscape context, we used U-series dating of speleothems to constrain the tectonic uplift of the Southern Mountains that caused the Solo River to divert north-ward to flow through the Kendeng Hills and form the Solo River strath terrace sequence (Fig.3, Extended Data Fig. 1). Second, to establish a terrace context, we applied red thermoluminescence, post-infrared infrared-stimulated luminescence (pIR-IRSL) and 40Ar/39Ar dating tech-niques to terrace sediments at localities covering the entire incision sequence: Kerek (upper), Padasmalang (middle), Ngandong (lower), Sembungan terrace (lower, contemporaneous with Ngandong), Ngle-back (lowermost) and Menden terrace (outside of the Kendeng Hills and the Solo terrace sequence). We used the terrace chronology to identify the incision phases and constrain the age of the Ngandong terrace by its relative position in the terrace sequence (Extended Data Fig. 2). Third, we established a fossil context by re-excavating the Ngandong bone bed (Extended Data Fig. 6, Supplementary Information section 2) to better understand the sedimentology of the terrace fill, the taphonomy of the Ngandong fossil assemblage and to provide context-supported datable material. We applied laser-ablation U-series analyses16 to 8 bovid teeth and 15 mammalian long bones (Supplementary Information section 7) and coupled US–ESR analyses to 3 additional fossil bovid teeth (Sup-plementary Information section 6).According to the U-series-derived speleothem chronology (Sup-plementary Table 13), the Gunung Sewu section of the Southern Mountains had been uplifted and the Solo River had been diverted by >500 ka, which places a maximum age on the formation of the terrace sequence. Consistent with this, the red thermoluminescence, pIR-IRSL and 40Ar/39Ar chronologies indicate that the sequence of strath terraces was formed between 316±28 and 31±6 ka (with a maximum age of 358±26 kyr), and are all chronologically consistent with their elevation (Extended Data Figs.2, 8, Supplementary Figs.1, 2, Supple-mentary Tables 6, 7, 12). 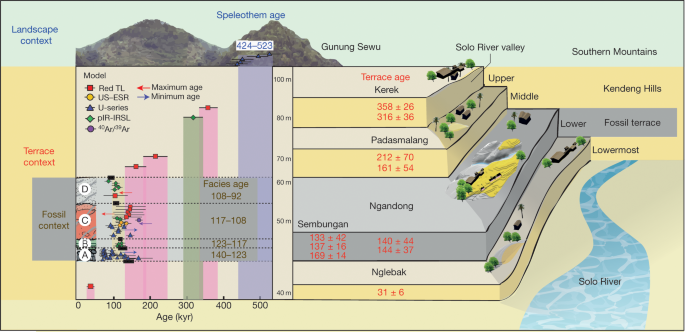 Fig. 3: A regional chronology for the Ngandong evidence, summarizing the results of our approach. Our Ngandong excavations yielded a composite cross-section with five lithofacies comprising the Ngandong terrace fill: these lithofacies could be related to terrace-fill descriptions from excavations at the site in the 1930s, which yielded the H. erectus calvaria and tibiae3,15 (Fig. 2, Extended Data Fig. 5, Supplementary Table 1, Supplementary Informa-tion section 2). All H. erectus fossils recovered in the original excavations were associated with facies C (gravelly sand bars). Our excavations yielded 867 in situ disarticulated non-hominin fossils—mostly isolated teeth and bone fragments, but largely complete elements were also observed3,17 (Extended Data Fig. 6c, f, g). Usually, these fossils were encased in a 10–25-cm-thick lens of poorly sorted fossil-rich muddy-pebble conglomerate that was deposited in a sediment-charged river (Fig. 2). Most of the 232 specimens that we analysed were broken before deposition, but more than 90% of them exhibit only minor abrasional rounding (Extended Data Fig. 7). Surface cracking and exfoliation indic-ative of substantial surface exposure is rare. No fossils show evidence of being reworked from a considerably older formation. Fossils from faciesA and faciesC have a similar taphonomy but fossils from facies C are larger, and more complete. No stone tools were discovered in this excavation. However, a concentration of 89 artefacts (dated to about 130 ka) was discovered in a contemporaneous Sembungan excavation located upstream of Ngandong (Extended Data Fig. 3, Supplementary Information section 3), and—when combined with the Elephas speci-men discovered in the older Menden terrace, located downstream of Ngandong (Extended Data Fig. 4, Supplementary Table 5, Supplemen-tary Information section 4)—this evidence provides an occupational context for the Ngandong hominins.Laser-ablation U-series analysis yielded minimum age estimates of about 120 to 80 kyr and about 140 to 60kyr for the mammalian long bones and eight bovid teeth, respectively (Extended Data Fig. 9, Sup-plementary Tables10, 11). US–ESR dating of three additional bovid teeth provided age estimates corresponding to 134–118 ka (Extended Data Fig. 10, Supplementary Tables 8, 9). These age estimates for the Ngandong fauna agree with the age of deposition of the Ngandong ter-race (144±37 to 111±9 kyr), the age of the upper and middle terraces (316 to 161kyr), the maximum age for the lower terrace at Sembungan (169±14 kyr) and the age of the lowermost terrace (31±6kyr), according to the red thermoluminescence, pIR-IRSL and 40Ar/39Ar dating. When these 52 dating results are entered into a Bayesian model (Fig. 3, Sup-plementary Tables 14, 15), an age range of 140–92 kyr is established for the deposition of the entire Ngandong terrace sequence, and a range of 117–108 kyr for the bone bed in facies C (Fig. 3), which contained the H. erectus material discovered in the 1930s.Our modelled age range for the Ngandong terrace is substantially older than the direct 230Th/234U determinations (40 to 60–70 kyr) that have been reported for the Ngandong H. erectus calvaria13, and is similar to previously reported combined U-series and ESR ages (correspond-ing to 77 to 143kyr) for faunal teeth in the terrace at Jigar14. However, the upper age limit for the Ngandong site (of about 500kyr) that was previously suggested on the basis of 40Ar/39Ar analyses of water-lain pumice from the Ngandong formation14, and the age for Ngandong H. erectus calvaria of >200 kyr based on gamma-spectrometric U-series techniques13, are at odds with our proposed age range for the bone bed (117–108 kyr) and our age for the next-oldest terrace in the sequence (162 ± 33 kyr). We believe that our bone-bed chronology is much closer to the actual age of Ngandong H. erectus.Sedimentary and taphonomic observations at Ngandong are best explained by a single flood event, during which facies B, C and D accumulated in rapid succession17 (Supplementary Information sec-tions 2, 10). A reliable age estimate for the formation of the facies and the non-hominin fossils at Ngandong establishes a depositional age for the H. erectus remains (Fig. 2, Supplementary Information sec-tions 5–9). The fossils show only incipient weathering and transport damage, despite being deposited in a river (Extended Data Fig. 7). 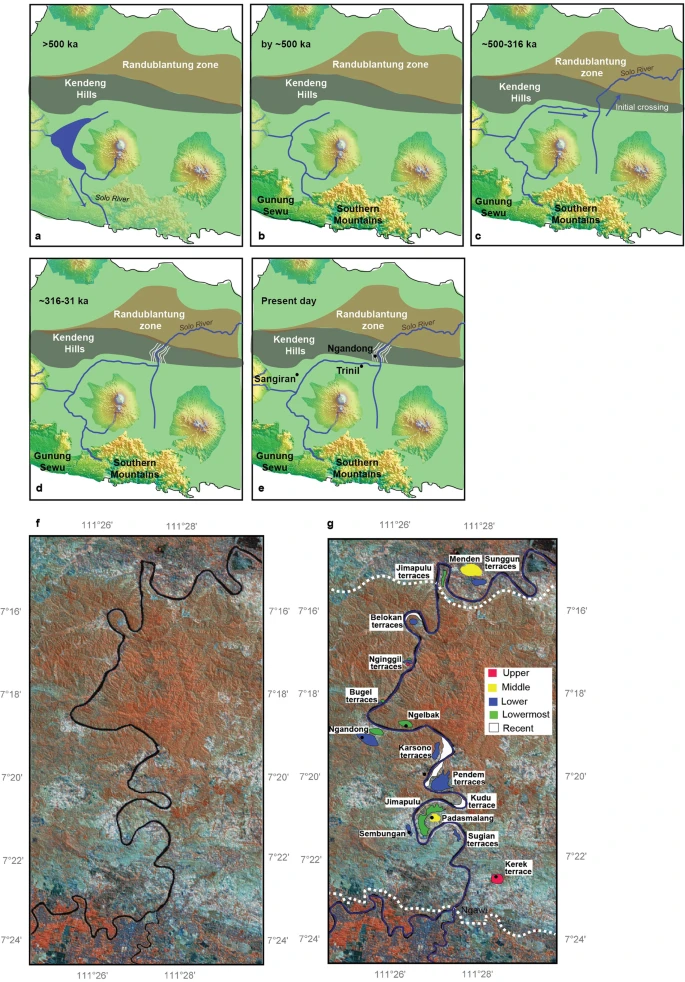 Extended Data Fig. 1 Evolutionary and geomorphical history of region. This dated sedimentary and taphonomic framework for the Ngan-dong bone bed does not support an overlap between modern humans and H. erectus in this region9,18,20. Instead, the Ngandong fauna in facies A (140±24ka) pre-dates the rainforest-associated site of Punung (between 128±15 and 118±3ka)22, which agrees with the proposed biostratigraphical sequence of Java based on the palaeoenvironmental and associated faunal changes23. The H. erectus bone bed (facies C) overlaps with Punung and falls within the sea level lowstand at the onset of termination II (around 120 ka)24. Thus, it represents the last, dying remnants of the archaic fauna and open woodland environments that were superseded by the impeding rainforest flora and fauna associated with Punung (Supplementary Information section 10). Furthermore, we can place Ngandong into a regional framework for Island Southeast Asia. H. erectus continuously inhabited the island, with dates on Java that start at 1.51 to 0.93 million years ago at Sangiran1,2, then 540 to 430ka at Trinil25 and ending with 117 to 108 ka at Ngandong. H. erectus was dispersed widely by 700 ka, as shown by archaeological evidence for hominins at Mata Menge (Flores, Indonesia)26 and Cagayan Valley (Luzon, Philippines)27. Two insular dwarf hominins are found on these outlying islands: Homo floresiensis at 100 to 60 ka28 and Homo luzonensis at 66.7±1ka29. Phylogenetic relationships have yet to be deter-mined for these two hominins, but they show morphological similarities with H. erectus29. Sharing similar temporal ranges, Ngandong H. erectus, H. floresiensis and H. luzonensis represent three evolutionary trajectories of Homo in Island Southeast Asia, each of which ended in extinction.Genomic evidence from modern populations in New Guinea provides estimates for the dates of the arrival of another early hominin in Island Southeast Asia. Two Denisovan lineages diverged from the Altai Den-isovans, one at about 363 ka and the other at about 283 ka30. These deep divergence dates provide evidence for the early arrival of Denisovans in Island Southeast Asia. Dispersing Homo sapiens encountered Den-isovan lineages in Island Southeast Asia at about 45.7 ka30 and at about 29.8 ka30. Additionally, a residual signal of approximately 1% archaic DNA in modern regional populations lies outside the human–Neander-thal–Denisovan clade30. This may reflect a past introgression event with H. erectus and provide evidence that these Denisovans encountered a late-surviving H. erectus population. An increasingly complex picture of hominin evolution in Pleistocene Island Southeast Asia is emerging from fossil and genomic evidence. The chronology of Ngandong H. erectus is critical for this narrative. We have approached the age of the Ngandong site in three increasingly precise contexts: the Kendeng Hills landscape, the Solo River terraces and the Ngandong bone bed. Our age estimates for the vertebrate fos-sils—including H. erectus—at Ngandong are, therefore, firmly anchored within their regional chronological and geomorphical contexts. With modelled ages of 117 to 108 kyr, the Ngandong bone bed can finally assume its correct position in the hominin biostratigraphical sequence of Island Southeast Asia. Rizal, Y., Westaway, K.E., Zaim, Y. et al. Last appearance of Homo erectus at Ngandong, Java, 117,000–108,000 years ago. Nature (2019) |
|


















