|
|
Post by Admin on Jun 29, 2016 22:40:34 GMT
Basal Eurasian ancestry was pervasive in the ancient Near East and associated with reduced Neanderthal ancestry 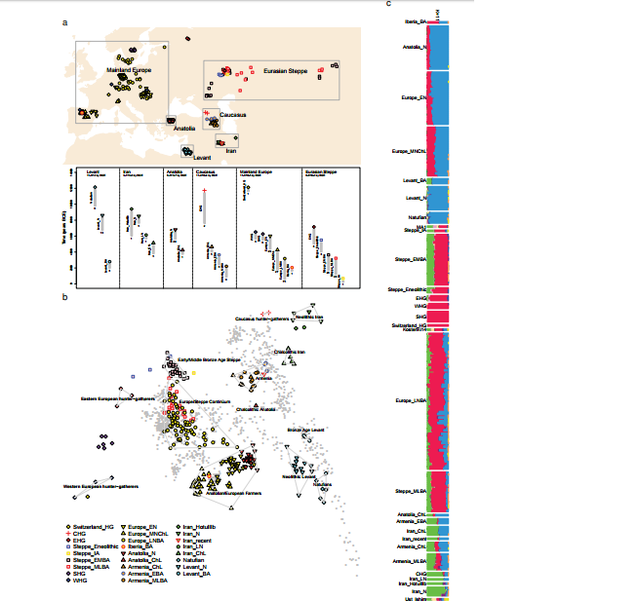 The ‘Basal Eurasians’ are a lineage hypothesized13 to have split off prior to the differentiation of all other Eurasian lineages, including both eastern non-African populations like the Han Chinese, and even the early diverged lineage represented by the genome sequence of the ~45,000 year old Upper Paleolithic Siberian from Ust’-Ishim11. To test for Basal Eurasian ancestry, we computed the statistic f4(Test, Han; Ust’-Ishim, Chimp) (Supplementary Information, section 4), which measures the excess of allele sharing of Ust’-Ishim with a variety of Test populations compared to Han as a baseline. This statistic is significantly negative (Z<-3.7) for all ancient Near Easterners as well as Neolithic and later Europeans, consistent with their having ancestry from a deeply divergent Eurasian lineage that separated from the ancestors of most Eurasians prior to the separation of Han and Ust’-Ishim. We used qpAdm7 164 to estimate Basal Eurasian ancestry in each Test population. We obtain the highest estimates in the earliest populations from both Iran (66±13% in the likely Mesolithic sample, 48±6% in Neolithic samples), and the Levant (44±8% in Epipaleolithic Natufians) (Fig. 2), showing that Basal Eurasian ancestry was widespread across the ancient Near East. 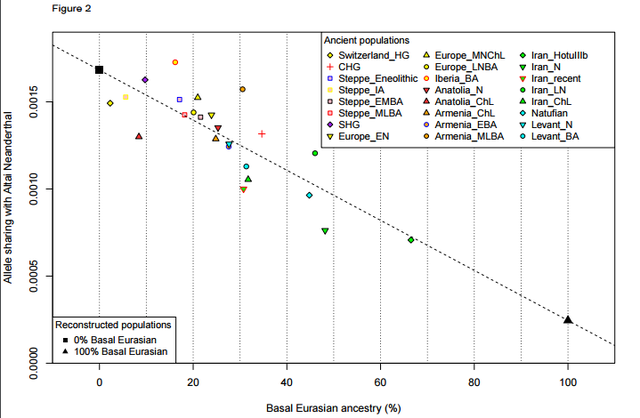 West Eurasians harbour significantly less Neanderthal ancestry than East Asians19,20-23, which could be explained if West Eurasians (but not East Asians) have partial ancestry from a source diluting their Neandertal inheritance21. Supporting this theory, we observe a negative correlation between Basal Eurasian ancestry and the rate of shared alleles with Neanderthals19 (Supplementary Information, section 5; Fig. 2). By extrapolation, we infer that the Basal Eurasian population had lower Neanderthal ancestry than non-Basal Eurasian populations and possibly none (ninety-five percent confidence interval truncated at zero of 0-60%; Fig. 2; Methods). The finding of little if any Neanderthal ancestry in Basal Eurasians could be explained if the Neanderthal admixture into modern humans 50,000-60,000 years ago11 largely occurred after the splitting of the Basal Eurasians from other non-Africans.  It is striking that the highest estimates of Basal Eurasian ancestry are from the Near East, given the hypothesis that it was there that most admixture between Neanderthals and modern humans occurred19,24. This could be explained if Basal Eurasians thoroughly admixed into the Near East before the time of the samples we analyzed but after the Neanderthal admixture.  A population without Neanderthal admixture, basal to other Eurasians, may have plausibly lived in Africa. Craniometric analyses have suggested that the Natufians may have migrated from north or sub-Saharan Africa25,26, a result that finds some support from Y chromosome analysis which shows that the Natufians and successor Levantine Neolithic populations carried haplogroup E, of likely ultimate African origin, which has not been detected in other ancient males from West Eurasia (Supplementary Information, section 6) 7,8. However, no affinity of Natufians to sub-Saharan Africans is evident in our genome-wide analysis, as present-day sub-Saharan Africans do not share more alleles with Natufians than with other ancient Eurasians (Extended Data Table 1). (We could not test for a link to present-day North Africans, who owe most of their ancestry to back-migration from Eurasia27,28.) The idea of Natufians as a vector for the movement of Basal Eurasian ancestry into the Near East is also not supported by our data, as the Basal Eurasian ancestry in the Natufians (44±8%) is consistent with stemming from the same population as that in the Neolithic and Mesolithic populations of Iran, and is not greater than in those populations (Supplementary Information,section 4). Further insight into the origins and legacy of the Natufians could come from comparison to Natufians from additional sites, and to ancient DNA from north Africa. doi: dx.doi.org/10.1101/059311 |
|
|
|
Post by Admin on Jul 9, 2016 22:44:35 GMT
 It is an open question whether archaic hominins’ deleterious mutation load contributed to their decline and extinction. However, there is clear evidence that Neanderthals escaped total genetic extinction by interbreeding with the anatomically modern humans who left Africa between 50,000 and 100,000 years ago (Green et al. 2010). In Europeans and Asians, haplotypes of Neanderthal origin have been inferred to compose 2–4% of each individual’s genome. When pooled together, these Neanderthal haplotypes collectively span ∼30% of the human reference sequence (Sankararaman et al. 2014; Vernot and Akey 2014). The introgression of Neanderthal alleles related to hair, skin pigmentation, and immunity appears to have provided non-Africans with adaptive benefits, perhaps because Neanderthals had preadapted to life in Europe for thousands of years before humans left Africa (Abi-Rached et al. 2011; Mendez et al. 2012; Sankararaman et al. 2014; Vernot and Akey 2014; Dannemann et al. 2016). However, these positively selected genes represent a tiny fraction of Neanderthal introgression’s genetic legacy. A larger number of Neanderthal alleles appear to have deleterious fitness effects, with putative links to various diseases as measured by genome-wide association studies (Sankararaman et al. 2014; Simonti et al. 2016).  Figure 1 The distribution of deleterious mutations in humans has been the subject of much recent research. A controversial question is whether the out-of-Africa bottleneck created differences in genetic load between modern human populations (Lohmueller 2014; Henn et al. 2015). Some previous studies concluded that this bottleneck saddled non-Africans with potentially damaging genetic variants that could affect disease incidence across the globe today (Lohmueller et al. 2008; Fu et al. 2013; Henn et al. 2016), while other studies have concluded that there is little difference in genetic load between Africans and non-Africans (Simons et al. 2014; Do et al. 2015). Although previous studies have devoted considerable attention to simulating the accumulation of deleterious mutations during the out-of-Africa bottleneck, none to our knowledge have incorporated the fitness effects of introgression from Neanderthals into non-Africans. In our simulation with additive fitness effects, the median Neanderthal was found to have fitness 0.63 compared to the median human (Figure 1A). Assuming recessive fitness effects, the excess load accumulated by Neanderthals was even greater, with a median Neanderthal fitness of 0.39 compared to the median human (Figure 1B). Such a large fitness disadvantage would have been incompatible with Neanderthal survival if they were competing with humans under conditions of reproductive isolation. In each case, the fitness differential was caused by accumulation of weakly deleterious mutations with selection coefficients ranging from (nearly neutral in the larger human population) to (nearly neutral in the smaller Neanderthal population). This agrees with asymptotic predictions that mutations with Embedded Image are not affected by a bottleneck with minimum population size N (Balick et al. 2015).  Figure 2 We performed between one and six replicate introgression simulations that began with the same parent populations but randomly generated different human/Neanderthal hybrids. A Neanderthal gene flow date of 2000 generations before the present is compatible with Fu et al.’s (2014) claim that the admixture occurred 52,000–58,000 years ago, assuming a human generation time between 26 and 29 years. To simulate the out-of-Africa bottleneck, which affected humans around the time of admixture, we used a model based on the history inferred by Gravel et al. (2011) from the site frequency spectrum of the 1000 Genomes data. The average allele frequency of these markers started out equal to the admixture fraction f, but was observed to increase over time. An initial admixture fraction of 1% was found to be consistent with a present-day admixture fraction ∼3%, with most of the increase occurring over the first 500 generations. The selection favoring Neanderthal alleles is an example of dominance heterosis (Davenport 1908; Shull 1914; Jones 1917; Crow 1948), selection for foreign DNA that is protective against standing deleterious variation.  Figure 3 Before admixture, most deleterious alleles are private to either humans or Neanderthals, leading introgressed Neanderthal alleles to be hidden from purifying selection when they are introduced at low frequency. Because Neanderthal haplotypes rarely have deleterious alleles at the same sites that human haplotypes do, they are protective against deleterious human variation, despite the fact that they have a much higher recessive burden than human haplotypes. It is worth noting that these simulation results assume random mating within Neanderthals and archaic humans; if consanguinity were widespread in either population, this could eliminate much recessive deleterious variation. However, if consanguinity were common in Neanderthals and rare in contemporary humans, a plausible scenario given Neanderthals’ smaller population size, we would still expect to see some positive selection for introgressed Neanderthal DNA. The strength of this selection should depend mostly on standing recessive variation within the human population, which would not be affected by Neanderthal inbreeding.  Figure 4 If most deleterious mutations have additive fitness effects instead of being recessive, different predictions emerge. The reduced fitness of Neanderthals is not hidden, but imposes selection against hybrids in the human population. Such selection against negative deleterious mutations could potentially be offset by positive selection or by associative overdominance due to linked recessive mutations. In the absence of these effects, however, we found that an initial admixture fraction of 10% Neanderthals was necessary to observe a realistic value of 2.5% Neanderthal ancestry after 2000 generations. Most of the selection against Neanderthal ancestry occurred within the first 20 generations after admixture, at which point the average frequency of the Neanderthal markers had already declined below 3% (Figure 4). During the first 20 generations the variance in admixture fraction between individuals is relatively high, permitting efficient selection against the individuals who have more Neanderthal ancestry than others. However, once all individuals have nearly the same admixture fraction but have retained Neanderthal DNA at different genomic locations, Hill–Robertson interference slows down the purging of foreign deleterious alleles (Hill and Robertson 1966; McVean and Charlesworth 2000; Roze and Barton 2006). This suggests that introgression of Neanderthal DNA into humans would have been possible without positive selection, despite the high mutational load, but would require a large initial admixture fraction, perhaps close to 10%. GENETICS June 1, 2016 vol. 203 no. 2 881-891; DOI: 10.1534/genetics.116.186890 |
|
|
|
Post by Admin on Jul 12, 2016 22:41:11 GMT
 Neanderthal bones from an excavation in Belgium have yielded evidence of intentional butchering. The findings, from the Goyet caves near Namur, are the first evidence of cannibalism among Neanderthals north of the Alps. The skeletal remains were radiocarbon-dated to an age of around 40,500 to 45,500 years. Remarkably, this group of late Neanderthals also used the bones of their kind as tools, which were used to shape other tools of stone. Professors Hervé Bocherens and Johannes Krause of Tübingen's Senckenberg Center for Human Evolution and Palaeoenvironment, along with Cosimo Posth and Christoph Wissing, also of the University of Tübingen, took part in the investigations. A review of the finds from the Troisième caverne of Goyet combined results from various disciplines; it identified 99 previously uncertain bone fragments as Neanderthal bones. That means Goyet has yielded the greatest amount of Neanderthal remains north of the Alps.  By making a complete analysis of the mitochondrial DNA of ten Neanderthals, the researchers doubled the existing genetic data on this species of humans which died out some 30,000 years ago. They confirmed earlier studies' results, which showed relatively little genetic variation in late European Neanderthals -- in other words, that they were closely related to one another. The findings have been published in the latest Scientific Reports. The Troisième caverne of Goyet was excavated nearly 150 years ago. Today, researchers are able to extract vast amounts of information using current methods -- such as precise digital measurement and categorization of the bones, examination of the conditions in which the bone fragments were preserved, as well as isotopic and genetic analysis.  Some Neanderthal remains from Goyet have been worked by human hands, as evidenced by cut marks, pits and notches. The researchers see this as an indication that the bodies from which they came were butchered. This appears to have been done thoroughly; the remains indicate processes of skinning, cutting up, and extraction of the bone marrow. "These indications allow us to assume that Neanderthals practised cannibalism," says Hervé Bocherens. But he adds that it is impossible to say whether the remains were butchered as part of some symbolic act, or whether the butchering was carried out simply for food. "The many remains of horses and reindeer found in Goyet were processed the same way," Bocherens says. Researchers have long debated the evidence of cannibalism among Neanderthals, which until now focused on the sites of El Sidrón and Zafarraya in Spain and two French sites, Moula-Guercy and Les Pradelles. The Troisième caverne of Goyet is the first example of this phenomenon from more northern parts of Europe. Four bones from Goyet clearly indicate that Neanderthals used their deceased relatives' bones as tools; one thigh bone and three shinbones were used to shape stone tools. Animal bones were frequently used as knapping tools. "That Neanderthal bones were used for this purpose -- that's something we had seen at very few sites, and nowhere as frequently as in Goyet," Bocherens says. |
|
|
|
Post by Admin on Jul 28, 2016 21:30:26 GMT
 Riparo Mezzena is a rockshelter located at ca. 200 m above sea level in the Monti Lessini mountain range, about 8 km from Verona in northern Italy (Fig. 1). The archaeological site was discovered in 1957 by Prof. Franco Mezzena, hence the site name9, and is one among several important Palaeolithic sites in the Monti Lessini territory, which also include Riparo Tagliente, Grotta della Ghiacciaia and Grotta di Fumane10,11,12. The archaeological stratigraphic sequence of Riparo Mezzena is composed of three layers9,13,14. The lowermost and middle layers, layers III and II respectively, contain diagnostic Mousterian lithic industries. These levels are overlain by layer I, which was found to contain a mixture of Palaeolithic and later pre- and proto-historic artefacts, including ceramics attributable to the Iron Age. During the study of the faunal assemblage by Angelo Pasa, nine bone fragments from layer I and four fragments from the other two layers were identified as human. These 13 remains include an incomplete mandible, 11 cranial fragments and one post-cranial bone fragment. A monograph by Corrain15 tentatively attributed the fragmentary mandible IGVR 203334 to a female Neanderthal, presumably based on the presence of Mousterian lithic industries in all three layers rather than on the size and morphology of the specimen, which displays possibly modern features (Fig. 2)15.  Figure 1: Location, view of entrance, stratigraphic sequence and the plan of Riparo Mezzena. During the last ten years, Monti Lessini human remains have been re-examined by means of anatomical and palaeogenetic analyses8,16,17,18,19,20. This work was complemented by a new study of the lithic assemblage21. Based on the amplification of the hypervariable region 1 of the mitochondrial genome by PCR16, one of the cranial fragments, MLS 1, was shown to carry Neanderthal-like mitochondrial DNA (mtDNA). The mitochondrial sequence of MLS 1 was reproduced in later studies, accompanied by the successful retrieval of short stretches of nuclear gene sequences from the same specimen18,20. In addition, retrieval of Neanderthal mitochondrial and nuclear DNA was also reported for MLS 319, and a short Neanderthal mtDNA sequence was obtained from the mandible8. Together these reports support the notion that the Monti Lessini material belongs to one Neanderthal individual. However, the evidence regarding the age of the material is based only on a single radiocarbon date (RTT-5578: 14C Age 34,540 ± 655; (68.2%) 39,870-38,420 calibrated years before present (cal BP); (95.4%) 40,780-37,480 cal BP) obtained on a bovid bone from layer III7. Unfortunately this faunal sample was not directly associated with the human remains of layer I, but came from the lowermost part of the Mezzena sequence. Nonetheless, its relatively young age in the broader context of the European Mousterian was interpreted as evidence for the presence of late-surviving Neanderthals in the Monti Lessini area7, possibly contemporaneous with anatomically modern human makers of Proto-Aurignacian industries from the neighbouring site of Fumane, dated between ca. 41,000 to 38,000 cal BP22. This, together with the genetic characterization of the human remains as Neanderthal, and the ambiguous anatomical features, led Condemi and collaborators8 to hypothesize interbreeding between Neanderthals and anatomically modern humans in this part of northern Italy.  Figure 2: Riparo Mezzena mandible. Ancient DNA DNA was extracted from nine Mezzena specimens, which included the five fragments that were directly radiocarbon dated and four fragments identified by ZooMS (Table 2). Between 9.6 mg and 21.6 mg of bone powder were removed from the specimens for DNA extraction33. DNA libraries were generated using a highly sensitive single-stranded library preparation method34, enriched for human mitochondrial DNA (mtDNA)35 and sequenced on Illumina’s MiSeq or HiSeq platforms. Full-length molecule sequences were reconstructed from overlapping paired-end reads and aligned to the revised Cambridge reference sequence of the human mtDNA genome (rCRS, NC_0120920) using BWA36. In total, we obtained between 2,774 and 7,903 unique mitochondrial sequences from each of the specimens (Supplementary Table S1). To determine whether some of these sequences are of ancient origin, we next established the frequency at which cytosines (C) in the reference genome are substituted by thymines (T) in each position of the aligned sequences. Elevated frequencies of C to T substitutions are expected to occur in genuine ancient DNA due to deamination of cytosines to uracils (U), particularly in single-stranded overhangs at the ends of DNA fragments37, and are largely absent in recent contamination38,39. Substantial signals of cytosine deamination were observed in four of the Mezzena specimens: IGVR 20334 (the mandible), IGVR 63017-15, IGVR 63017-3 and IGVR 63017-14 (Supplementary Table S1). These frequencies increase substantially when filtering for sequences that have a C to T substitution at the opposing end (Supplementary Table 1), an observation that is consistent with the presence of both endogenous ancient DNA as well as present-day human contamination in the specimens40,41. We did not find any evidence for ancient human DNA preservation in the remaining five specimens, including those identified as Suidae and Cervid/Saiga by ZooMS.  Figure 3: Assessment of the phylogenetic position of the Mezzena specimens IGVR 20334, IGVR 63017-15, IGVR 63017-3 and IGVR 63017-14 in the hominin mitochondrial tree based on phylogenetically informative (‘diagnostic’) positions. Since too few putatively deaminated sequences are available to reconstruct complete mitochondrial genomes, we focused our analyses on sequences overlapping phylogenetically informative sites in the mtDNA genome. In a first branch-specific analysis, we looked at sites where the mtDNA genomes of modern humans, Neanderthals, and the Denisovan/Sima de los Huesos (SH) clade share derived variants that set them apart from all of the other hominin groups and the chimpanzee41. All of the sequences that overlap positions that are derived only in Neanderthals support the ancestral, i.e. non-Neanderthal state (Fig. 3). Likewise, there are no sequences supporting the Denisovan/SH state. We detect, however, strong support (100%) for the modern human-specific state in the sequences from all four specimens. The discrepancies between the results of the genetic analyses performed here and in previous studies8,19 are striking. The fact that we did not detect authentic ancient DNA in MLS 3 using the most sensitive method currently available33 is difficult to reconcile with the presence of ancient DNA in the specimen. Moreover, the mandible, which exhibits poor but detectable levels of ancient DNA preservation in our analysis, carries mtDNA of the modern human type. It is important to note that previous work relied on amplification of short stretches of DNA by PCR, an approach that is much less sensitive than current library preparation and high-throughput sequencing techniques33. Unlike PCR, library preparation allows molecules to be sequenced in their entirety, thereby providing information on DNA degradation patterns that lend evidence to the ancient origin of the modern human sequences retrieved from the Mezzena mandible. Our results highlight once more that PCR-based ancient DNA analyses are prone to contamination43. Yet, the case of Mezzena is unusual in that contamination must have been repeatedly introduced through PCR products of Neanderthal DNA rather than genomic DNA from modern humans. It is unfortunate that MLS 1, the specimen studied most extensively by means of genetics16,18,20, is not available for repeated analyses. The fact that the published mtDNA sequence of MLS 1 differs from the sequences of other Neanderthals44 does not per se prove its authenticity. The MLS 1 sequence was reconstructed from several short PCR products and it is conceivable that it represents a patchwork of contaminant Neanderthal and modern human sequences rather than a genuine Neanderthal sequence. Our findings thus put a question mark over all previous genetic results obtained from the Mezzena remains. Based on the concordant results of the suite of techniques employed in our study, we do not support the hypothesis put forward by Condemi and collaborators8 that Riparo Mezzena and its surroundings was an area of long chronological overlap, where interbreeding between Neanderthals and anatomically modern humans took place. New excavations are required to gain a better understanding of crucial periods, such as the Middle-to-Upper Palaeolithic transition. If materials from sites excavated long ago (e.g. Riparo Mezzena) are to be used to provide additional data on these complex phases of our evolutionary history, then it should only be done using the whole suite of state-of-the-art methods at our disposal. Scientific Reports 6, Article number: 29144 (2016) |
|
|
|
Post by Admin on Aug 2, 2016 22:44:57 GMT
 El Sidrón Site The accidental unearthing in 1994 of an outstanding set of human fossils gave rise to the archeological excavation and multidisciplinary study of the site (11). As a result, a significant archeopaleontological record is being recovered, largely composed of Neandertal remains. The karstic site of El Sidrón is located in the region of Asturias, Spain, (43°23′01″N, 5°19′ 44″W) (Fig. 1). Geologically, the site is found in the so-called “Surco Oviedo-Infiesto” (11), a strip of Mesozoic and Cenozoic sediments limited by Paleozoic relief to the north and south. The cavity is a pression tube of ≈600 m long, with a central stretch of 200 m oriented nearly west–east (“Galería del Río”). This tube shows on its southern bank transverse galleries in a NE–SW to N–S direction, generally of a restricted nature. The fossil site is located in one of these transverse galleries, the Osario Gallery, of ≈28 m long and 12 m at its widest part (Fig. 1). All of the remains are recovered from a surface ≤6 m2 within stratum III of the sedimentary sequence. Sediments accumulated in the Osario Gallery constitute a relatively thin deposit, so far prospected at a maximum thickness of 227 cm. The archeological assemblage recovered at the site is in secondary position, and it certainly comes from a close exterior location. Ex hypothesis, the original deposit was located outside the cavity, possibly in a doline close to the vertical of the site. A collapse of nearby fissures produced the sudden entry of the archeological material in a single event. Several taphonomic signals help to clarify the scenario. Refitting of several bone fragments and 53 stone tools indicates a limited displacement as well as synchrony of the assemblage. Preservation of osteological surfaces is excellent with very limited trampling and erosion. There are no toothmarks of large carnivores on the bones, with marginal action of rodents and a small carnivore (e.g., fox) on a few nonhuman remains. A few bones have hydraulic abrasion but no weathering. In short, the data point to a limited exposure of bones outside the cavity; a mass displacement moved the archeological deposit into the cave secondarily, with very little movement after final deposition.  Morphological Affinities of the Human Remains The El Sidrón teeth are large, with crenulated enamel and accessory cusps. Neandertal lineage incisive features (16) observed in the sample include shovel-shaping, marked labial convexity, and strongly developed lingual tubercles. On the premolars (17), an asymmetric lingual contour, strong transverse crests, a metaconid lingually located, and accessory lingual cusps are present (e.g., SD-763). The posterior dentition shows some cases of a noticeable taurodontism (e.g., SD-531). No upper face skeletal remains have been recovered, but three mandibles are well preserved (Fig. 2). The mandibular body tends to be high and thick. The mental trigone is strongly developed without any sign of a submental notch. Interestingly, the retromolar space is short in these mandibles. Other characteristic Neandertal lineage features include mental foramen below M1, deep pterygoid fossa, and inclined mylohoid line.  The neurocranium is well represented in the sample but fragmentary. Overall, the anatomy of these fossils corresponds to the set of features detected in Late Pleistocene Neandertals. The SD-436 frontal preserves a portion of the right squama and part of the supraorbital torus, with the superciliary region preserved. It shows a marked anterior projection with the development of a supraglabellar fossa, and the supratoral sulcus is well defined; there is a rounded torus with apparent lateral continuity among the three elements and a high degree of pneumatization reaching the lateral trigone. SD-438 is an immature right supraorbital torus and a portion of the squama. It shows a marked projection of the supraorbital torus and a clear supratoral sulcus. The temporal bones (SD-315 and SD-359) are still covered by concretions, but several diagnostic features (18) can be distinguished, including a low projection of the mastoid process, flattened glenoid fossa, and an inclined anterior wall of this fossa. Two occipital bones have been recovered. SD-1219 is a reasonably complete occipitomastoid region (Fig. 3), with the upper occipital scale and temporal petrosal in good condition but a badly fragmented basilar part. The occipital is large, with a marked nuchal torus and open sutures connecting with a well preserved temporal pyramid. A large suprainiac fossa is present (Fig. 3). SD-1149 is smaller and partially covered by thin breccia. Right transverse sinuses are observed in both cases.  Fig. 4. Mandibular measurements and indices suggesting geographic patterning in Neandertal populations. (Left and Center) Box plots of significantly different variables in a north–south geographic polarity. Box plots provide means, mean ± SE (box), and mean ± SD (whiskers). El Sidrón Human Remains and Neandertal Geographic Variation The evolutionary place of El Sidrón Neandertals has been investigated in the context of possible Neandertal geographic patterning. The mandible, as an anatomical system with clear European lineage-derived features, has been largely used, maximizing the intra-El Sidrón variability (n = 3). The comparative samples were divided into regional subgroups according to north–south and east–west geographic polarities. Specimens coming from the southwest Asia and south European peninsulas (Iberian, Italian, and Balkans) are included in the southern subset. The east–west boundary is established by the Adriatic Sea, with the Balkans in the east. Pairwise t test comparisons did not show differences between east and west. By contrast, a number of variables showed significant differences in a north–south division (Fig. 4). The position of the M3 in relation to other mandibular structures emerges as a determinant for the differences. Bi-M3 arcade width (t = 2,2711; df =14; P < 0.03) and M3–lingula length (t = −2,1964; df = 19; P < 0.04) show significant differences in a north–south polarity. Appreciable differences also hold for a variety of indices relating the latter variables with the M3–mental foramen length and corpus height (Fig. 4) (e.g., M3–lingula/M3–mental foramen, t test nonsignificant). Southern mandibles are wider at the level of the M3 and the coronoid process, and the corpus is located relatively closer to the ramus (e.g., shorter retromolar spaces). By contrast, northern mandibles are narrower, and the corpus–ramus distance is larger. This geographic patterning signal was further tested by geometric morphometrics.  Fig. 5. Thin-plate spline transformations of the mean shape into a predicted shape according to singular vector scores of a north–south axis. (A) Partial least-squares analysis. (B) 3D mean shape differences between northern and southern Neandertal samples. Presently, most of the scholars agree that Neandertals constitute a distinct human evolutionary lineage not involved in the initial ancestry of modern humans. The causes promoting the Neandertal anatomical pattern, nevertheless, are still under debate (37⇓–39). Climatic adaptation is a major factor invoked to accounting for most of the Neandertal anatomy (40, 41), although biomechanical adaptations (42, 43) and stochastic genetic processes (44) are raised for the explanation of specific traits. Clarification of these aspects has focused the research interest on the evolution of this archaic human group, and the amount of interbreeding between Neandertals and early modern humans after the arrival of the latter to Europe is a matter of contention (45⇓–47). Given the establishment of a Neandertal evolutionary lineage in Europe, we are beginning to address issues regarding the population history of Neandertals sensu stricto. Genealogical analyses of the ancient mtDNA sequences are showing well defined genetic groups, suggesting the existence of different lineages within the Neandertal gene pool (8, 9). In the context of Quaternary ecoclimatic instability, the evolution of Neandertals has been long enough to produce regional diversity of populations. If Middle Paleolithic human populations behaved as part of the Paleartic bioma, it would not be surprising if they exhibited the marked north–south faunal provinciality detected in Europe at least during the OIS 3 (48). Neandertal populations were largely isolated by geographic barriers (52⇓⇓–55), and at the peak of glacial events the European population was mainly concentrated in the south of the continent (54). In this framework, two properties should be expected for the southern populations. First, they should have had a larger temporal continuity, and in consequence more time for developing morphological variants. Second, a larger amount of variability should be found in these populations. The first expectation may be substantiated by the fact that northern samples are morphologically more similar to those from the Middle Pleistocene; that is, Neandertal populations from the north would maintain a more primitive condition within the European lineage. If so, the slightly distinctive morphology of southern samples may be interpreted as derived. Yet, southern samples (e.g., Krapina, El Sidrón) illustrate local variation that might be explored from this perspective. Published online before print December 12, 2006, doi: 10.1073/pnas.0609662104 |
|

























