|
|
Post by Admin on Mar 28, 2017 20:00:42 GMT
 Fig. 1. (A) Geographical location of Gruta da Aroeira and main sites mentioned in the text. (B) Detail of the excavation area and provenance of the ARO2 U-series sample. (C) Stratigraphic profile and cranium provenance (denoted by its field inventory no. 606). The Gruta da Aroeira Site Ongoing research and excavations since 1987 at the Almonda cluster of paleoanthropological localities in central Portugal (Fig. 1, Fig. S1, and SI The Gruta da Aroeira Site) have yielded human remains and rich archaeological levels of the Lower, Middle, and Upper Paleolithic as well as Early Neolithic and later prehistoric periods (1⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓–12). Within the Almonda karst system, the Gruta da Aroeira was first investigated from 1998–2002 (13), revealing a rich lithic assemblage with Acheulean bifaces (Fig. S2) associated with faunal remains and two human teeth (Fig. S3): Aroeira 1 (a left mandibular canine) and Aroeira 2 (a left maxillary third molar). Aroeira 1 is moderately large, especially compared with the Atapuerca (SH) sample, and Aroeira 2 is among the larger of the Middle Pleistocene upper right third molars (6, 14). They fit morphologically within the known variation of European Middle Pleistocene dentitions, although Aroeira 2 has a relatively large hypocone (6, 15).  Fig. S1. (A and E) Almonda escarpment with the position of Gruta da Aroeira and the Almonda River in the foreground. (B) General view of Gruta da Aroeira and the location of the main dated speleothems. (C) Estremadura Limestone Massif with the position of the Almonda spring, the Tagus River, and the Serra d’Aire. (D) Schematic cross-section of the Almonda karst system. (F) Acheulean biface (flint) from level Xb/c. (G) Gruta da Aroeira site plan. The Aroeira stratigraphy spans a thickness of ∼4 m and comprises three major stratigraphic units (Fig. 2 and SI Stratigraphic Outline). The cranium was recovered from unit 2, a 2.2-m-thick mud-supported breccia rich in angular and subangular clasts. This unit corresponds to the Acheulean layer X–Xb/c [the upper and lower parts of a single layer excavated in 1998–2002 (X) and in 2013–2015 (Xb/c)], and the overlying ARO2 flowstone has yielded a minimum age of 417.7 + 37.3/−27.5 ka (SI U-Series Results and Table S1) (9). A further uranium-thorium (U-Th) age of 406 ± 30 ka for the outer layer of a stalagmitic column (SI Chronostratigraphy) covered by unit 2 provides a maximum age for the sequence and allows correlation of it to Marine Isotope Stage (MIS) 11. Two additional U-Th ages of 390 ± 14 ka and 408 ± 18 ka for calcitic crusts that formed on the cranium provide additional, consistent minimum age constraints for the cranium itself (SI Chronostratigraphy). Thus, the Aroeira 3 cranium most likely dates to 390–436 ka.  Fig. 3. The original completely restored Aroeira 3 cranium in lateral view (A) and the virtual reconstruction and original fossil in inferior view (B) (also Figs. S4 and S5). (Scale bars, 5 cm.) The Aroeira 3 Cranium Preservation. The Aroeira 3 cranium was painstakingly extracted from the hard calcareous breccia and restored (SI In Situ Extraction of the Fossil and Fig. S4). The cranium is taphonomically broken obliquely to the sagittal plane, with the preserved bone margin running diagonally from the left supraorbital arch anteriorly, crossing the midline just anterior to bregma, and continuing posteriorly toward the right asterion. Approximately half of the right parietal bone and the right half of the frontal bone are preserved. A circular portion of the right frontoparietal region was originally present but was destroyed in situ in the act of discovery (Fig. 3). In addition, portions of the sphenoid and the nearly complete temporal bone are preserved, as well as the medial portion of the left supraorbital arch, the interorbital pillar (including the superior portions of both nasal bones), and most of the right supraorbital arch. The outer surface of the supraorbital torus is preserved only in the glabellar region and in the medial half of the right supraorbital arch (we use the term “supraorbital arch” to refer to the part of the supraorbital torus lateral to the glabellar region; thus, it comprises the supercilliary arch, the supraorbital margin, and the lateral trigone). In addition, a fragment of the right maxilla includes the lower border of the nasal aperture and a part of the anterior nasal floor. A small portion of the alveolar process of the right maxilla is also present, with two fragmentary molars partially preserved.  Fig. S4. Various stages during the in situ extraction and restoration process of the fossil, and reconstruction of the Aroeira 3 cranium after cleaning. (A) Outline of the cranium embedded in the breccia. (B) Location of the cranium after its protection with gauze coating and polyurethane resin. (C) Cutting of the breccia with a circular saw to remove the cranium. (D) Protection of the fossil with wooden boards during the fracturing of the speleothem adhering to the wall with a pneumatic hammer. (E) Final stage of the extraction of the cranium. Wooden boards were placed along both sides of the wall while the breccia block containing the cranium was cut along the bottom. (F) Main portion of the cranium embedded within the breccia block. (G) Isolated cranial fragments before restoration. (H) Main portion of the cranium during the removal of the hardened sediments from the endocranial surface. (I) Main portion of the cranium during the cleaning process (Left) and reconstruction of some of the isolated cranial fragments (Right). (J) Detail of the cranial base and temporal bone during the cleaning process. The arrow indicates a thin layer of speleothem coating which remains on the superior portion of the petrous pyramid. (K) Fragments comprising the cranium before reconstruction. (L) Manually joining the fragments together with adhesive. (M) Endocranial view of the reconstructed cranium. (N) Lateral view of the reconstructed cranium. (Scale bars, 5 cm.) Images in K–N are from J. Trueba (photographer). The cranial landmarks nasion, glabella, right asterion, right auriculare, and right porion are preserved. Although the bregma is not preserved, its position can be estimated accurately, because the coronal suture is preserved up to a point very close to the bregma. A remnant of the metopic suture is preserved near the glabella, as well as the right parietomastoid and occipitomastoid sutures and the segment of the lambdoid suture on the right parietal bone closer to the asterion. Internally, the frontal crest, foramen cecum, and crista galli are preserved in the anterior cranial fossa. |
|
|
|
Post by Admin on Mar 29, 2017 20:02:03 GMT
 Fig. S5. Virtual reconstruction of the Aroeira 3 cranium in frontal (A), posterior (B), superior (C), and endocranial (D) views. The frontal sinus in D is exposed in a parasagittal section located 4 mm to the right of the sagittal plane. (E) Virtual reconstruction of the Aroeira 3 cranium in a three-quarters view compared with Bilzingsleben B1 (cast). Supraorbital Region. Two main supraorbital torus morphologies can be found in European Middle Pleistocene fossils. In many of them, the supraorbital arches are curved mediolaterally (in frontal view) and rounded on their anterior surface. The two arches can fuse completely in a swollen glabellar region (Fig. 3 and Fig. S5), or they can remain more or less separated in the midplane by a glabellar depression. This supraorbital morphology, with different degrees of glabellar fusion, is found in the large Atapuerca Sima de los Huesos (SH) sample, in the Bilzingsleben B1, Steinheim, and Petralona crania, and in the Late Pleistocene Neandertals (16). It is also seen in the Early Pleistocene Atapuerca Gran Dolina specimen ATD6-15. However, the Arago 21 and Ceprano specimens depart from this condition, resembling the Middle Pleistocene African specimens from Kabwe and Bodo in which the two supraorbital arches are well separated at the glabella and are flatter and less curved (17, 18). Despite the loss of the outer surface over much of the supraorbital torus, it is clear to us that the supraorbital arches in Aroeira 3 are fused in a swollen glabella (i.e., unlike the Ceprano and Arago 21 crania, the supraorbital torus in Aroeira 3 is not medially concave). Although the precise morphology of the supraorbital arches is more difficult to assess, the better-preserved right side seems to show a rounded condition, and the Bilzingsleben B1 specimen represents the closest Middle Pleistocene match to the Aroeira 3 supraorbital torus (Fig. S5). Numerous Middle Pleistocene fossils, including the Kabwe, Bodo, Arago 21, and Petralona specimens (and perhaps the Steinheim specimen, although it has some deformation in this region) exhibit a nasion that is depressed with respect to glabella. In contrast, Neandertals and the Atapuerca (SH) sample show a nasion that projects to the same degree as glabella. The Aroeira 3 cranium, like the Bilzingsleben B1 specimen, is intermediate in this trait (Figs. S5–S7).  Fig. S6. The Aroeira 3 cranium compared with Atapuerca SH Cranium 4 and Cranium 5. Atapuerca SH Cranium 5 is oriented in the Frankfurt horizontal orientation; this orientation is estimated for the Aroeira 3 cranium and Atapuerca SH Cranium 4. Despite some abrasion of the outer surface, the right and left supraorbital arches are thick as compared with the majority of the European or African Middle or Late Pleistocene fossils (16, 19). The maximum midorbit thickness of the torus (19.0 mm) can be taken on the right side and is similar to that of the Bodo and Ceprano crania (each 17.5 mm), although the torus of the Bilzingsleben B1 cranium (21–22 mm, right side, cast measurement) is even thicker. The interorbital pillar is very broad. Although the dacryon and maxillofrontale landmarks cannot be located precisely, the distance between the two inner orbital borders is large (34–35 mm) and similar to the Atapuerca SH Cranium 4 (38.0 mm) and the Kabwe (32.0 mm), Bodo (37.5 mm), and Bilzingsleben B1 (35.5 mm, on cast) specimens. The frontal sinuses in the Aroeira 3 cranium are well developed (Fig. S7) but are not as large laterally (in the torus) or superiorly (in the frontal squama) as in the Petralona specimen.  Fig. S7. The Aroeira 3 cranium compared with Atapuerca SH Cranium 5 and the Steinheim specimen. The Frankfurt horizontal orientation is estimated in both the Aroeira 3 cranuim and the Steinheim specimen. The nasion seems depressed relative to the glabella in the Steinheim cranium, but the strong deformation of this specimen precludes a conclusive assessment. Virtual Reconstruction. Virtual reconstruction of the Aroeira 3 cranium by mirror-imaging the right side (Fig. S5) shows that the parietal walls are nearly vertical. However, the maximum cranial breadth is located at the supramastoid crest, as in other earlier Middle Pleistocene European fossils. This morphology departs from the ancestral condition seen in Homo erectus of strongly convergent parietal walls superiorly and also from the more circular contour in posterior view of late Middle and Late Pleistocene Neandertals (16). At present three different cranial morphologies can be recognized in the European Middle Pleistocene hominin record. One is almost fully Neandertal, with nearly the entire suite of derived traits, and occurs during the last part of this period, mainly <200 ka. A second cranial configuration shows many Neandertal traits in the face, supraorbital torus, temporal bone, and mandible, but the general shape of the neurocranium (in both lateral and posterior views) is not Neandertal-like, indicating a mosaic nature for Neandertal cranial evolution.  Finally, there are other European partial crania, such as the Arago 21 and Ceprano specimens, that do not show Neandertal-derived traits in the preserved regions or in which the features are more ambiguous (36). The Aroeira 3 cranium resembles these specimens in its well-developed angular torus (also present in Atapuerca SH Cranium 4) and its lack of a flattened articular eminence. Although the taxonomic identity of the Arago and Ceprano hominins is debated, some authors prefer to group them with other Middle Pleistocene fossils from Africa and Asia in a separate species (Homo heidelbergensis) (34, 40). This view sees the Neandertals as evolving out of H. heidelbergensis in Europe and posits a largely anagenetic (linear) evolutionary scenario. Other researchers (23) have argued for a high degree of morphological diversity in the Middle Pleistocene European hominin record, a scenario that is incompatible with an anagenetic evolutionary pattern. Evolutionary scenarios that posit a series of temporally successive grade shifts are likely to be largely a product of the general paucity and poor chronological control of the European Middle Pleistocene fossil record. Elucidating nonlinear evolutionary patterns in the hominin fossil record relies on fossil morphology, as well as geography and chronology, and the addition of relatively complete, well-dated fossils, such as the Aroeira 3 specimen, will help establish a more robust evolutionary scenario. Published online before print March 13, 2017, doi: 10.1073/pnas.1619040114 PNAS March 28, 2017 vol. 114 no. 13 3397-3402 |
|
|
|
Post by Admin on Apr 11, 2017 19:51:23 GMT
Around 50,000 years ago in Spain, a Neanderthal had a toothache and popped the botanical version of an aspirin. Maybe. Although it's far from clear-cut, there’s evidence from old teeth that hints at the possibility. It's part of a study of Neanderthal diet, courtesy of their poor dental hygiene. Published in Nature, an analysis of preserved dental plaque from three different Neanderthals provides an intriguing glimpse into what they put in their mouths. According to the authors, the analysis points to regionally varied diets and suggests possible medicinal plant use.  But some of the DNA evidence is a little strange, suggesting evidence of species where they really shouldn’t have been 50,000 years ago. There are some good explanations for why this could happen, but, like most exciting results, drawing conclusions from the evidence demands a little caution. Previous research has found that the wear patterns on their teeth suggest a varied diet with regional differences. And dental plaque has been used before to analyze the starches and proteins that were preserved in the plaque. These analyses suggest that Neanderthals were eating many plants, possibly including medicinal ones. 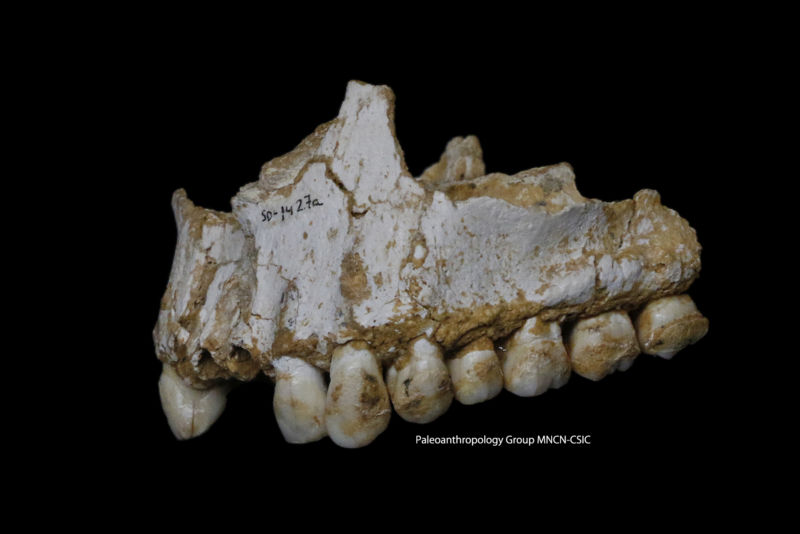 But dental plaque can preserve more than simple chemicals; genetic material from the food can be encased in it. This allowed a team of researchers, led by Laura Weyrich at the University of Adelaide, to get an incredibly detailed look at what plant and animal species three individual Neanderthals had been eating. Two were from El Sidrón Cave in Spain, including the potential aspirin-popper, while one was from Spy Cave in Belgium. The results add to previous evidence suggesting that the “Neanderthal diet” was actually many different things, depending on where the Neanderthals in question lived. The Belgian followed the meat-heavy pattern, with genetic material from woolly rhinoceros, mushrooms, and wild sheep showing up in the dental plaque. Mammoth, reindeer, rhinoceros and horse bones in the cave tell the same story as the dental plaque: these were hunters.  They found that there were different groupings of oral microbiomes: the Spanish Neanderthals grouped with chimpanzees and ancient African gatherers, in what the researchers called a “forager-gatherer” group with a largely vegetarian diet. The Belgian Neanderthal grouped more closely with the typical meat-heavy hunter-gatherer diet. A modern human and early agriculturalist human also had different profiles. The result helps us understand modern human oral microbiomes in context, says Dobney. Our current food-related health problems, like obesity, didn’t happen in a vacuum: “it hasn’t happened overnight; it’s part of the journey that we’ve been on for thousands of years. Major cultural changes like the beginnings of agriculture are still impacting our health today.” As for Neanderthals, he hopes evidence of their important place in our own history—in terms of behavior, genome, and microbiome—can help end the common perception of them as “these knuckle-dragging cavemen able to do not much more than bring down the odd bison here and there.” The evidence is pointing toward varied behavior across the Neanderthals, and we’re starting to be able to get closer to inferences about the sophistication of their behavior and culture. |
|
|
|
Post by Admin on Apr 22, 2017 20:00:58 GMT
 Somewhere between 1.5 and 2.1 percent of your genome was inherited from the Neanderthals, assuming your ancestry was non-African of course. East Asians typically have more Neanderthal DNA because their ancestors partook in a little more afternoon delight than the rest of ours did.  For Indigenous people living around eastern Indonesia, and in New Guinea and Australia, their ancestors also took a shine to the 'Denisovans'. In their genomes we find an extra 4 to 6 percent inherited from this mysterious species. So far, archaeologists have found just two finger bones and a tooth from the Denisovans, thousands of kilometres away from New Guinea in southern Siberia, of all places.  Yet, the fact that the earliest New Guineans mated with the Denisovans only 44,000 years ago - as revealed by their DNA - suggests that all the action happened in the tropical climes of Oceania, not icy Siberia. Neanderthal DNA is associated with an increased risk of developing skin corns and callosities, mood disorders and depression, overweight and obesity, upper respiratory and urinary tract infections, incontinence, hardening of the arteries and even smoking. |
|
|
|
Post by Admin on Apr 29, 2017 19:50:18 GMT
Toronto novelist Claire Cameron, whose 2014 bestseller The Bear took place in the contemporary Canadian wilderness, moves across time and space to an equally dangerous locale for The Last Neanderthal, set in France 40,000 years ago. The intricate tale of Girl, the title character, and Rose, the modern-day archaeologist who discovers her bones, is a story of common humanity, including the fact that the more things change for pregnant women, the more they stay the same. The novel is also thoroughly immersed in the recent explosion in knowledge—and speculation—about our closest kin. Cameron spoke with Maclean’s about the lives and fate of the Neanderthals. Q: You note that Neanderthal—“stooped over, hairy, primitive, dull”—is still an insult in use, but our cousins have been picking up a lot of good press lately. Why are we so fascinated? A: Since 2010, yes, and the first draft of the genome. That really changed the perception. They’re almost like a Shakespearean foil now. We can see ourselves in them. Q: And we did see them, literally. That makes a difference. A: It’s the interbreeding between the two groups that really takes it up a notch: between one and four per cent of European and Asian DNA is Neanderthal. Q: So, as usual, it’s all about us. What did we get out of this genome infusion? Red hair, which Girl has, has caught the popular imagination. A: We are a self-centred storytelling species, aren’t we? Yes, there’s the red hair, though that comes from a small sample. Scientists are starting to look into things like immunity. For genes to stick around, they do need to play some sort of role often—not always—but there are all sorts of things in the news at the moment about what and why.  Q: Red hair, herpes and bad skin, I’ve seen. A: Yes, quite negative, but also protection against schizophrenia. Q: They were clearly as caring, as witnessed by their burials, and much more flexible and adaptive than we once thought. A: Neanderthals were tremendously successful for 200,000 years, through all kinds of climate change—longer than us, so far. There’s a recent study about the plaque on their teeth, and one group in Spain had a vegetarian diet. Amazing. The point is that Neanderthals weren’t one thing. They were people spread all over Europe and Asia in all kinds of environments and they were as varied in their responses as we are. Q: The other side of “all about us” is the idea of modern humans as the doom of the Neanderthals, either by deliberate violence or simply by showing up and ruining their world. What is the thinking on that now? A: I’m by no means an expert, but I did go through all the new research. I think that their extinction is tied to low population—humans moving into their territory was one more pressure on their food sources. Disease, genetics, all those things that exert pressure on large predators pushed them [to a tipping point]. There were a lot of extinctions of larger mammals around their time. We no longer have giant sloths or cave bears.  Q: It can’t be coincidental that we were on the scene at the time of their demise, though, because we certainly weren’t ignoring them. The kind of permanent effect on our genome must have meant massive, ongoing gene flow—as sex is politely called—between us. A: The genome sequencing came from two bones from two places. You only find as much DNA as there is in your sources. So I don’t think we have a full picture yet of what was going on, but there may have been more interbreeding than originally thought; it’s one of those older assumptions that I think is changing. Our ability to think in tens of thousands of years is limited, too. That’s certainly one thing I learned about myself writing this book. Human migration is as messy as humans are. The fairly simple out-of-Africa story is getting complicated—the DNA shows we co-evolved alongside Neanderthals, rather than after them. Q: Close contact doesn’t mean always peaceful. A: I had a big discussion with Yuval Noah Harari, who wrote Sapiens: A Brief History of Humankind, after he read a draft of my novel. He said, “You know, conflict with humans doesn’t come into this.” I thought a lot about that, and I’m sure there was conflict between Neanderthals and humans. I’m sure there were also friendships and relationships as well. That’s my speculation: it’s going to very much depend on who is under threat and who is under pressure and the circumstances under which you meet and then the individual personalities involved. |
|
















