|
|
Post by Admin on Dec 12, 2017 19:29:01 GMT
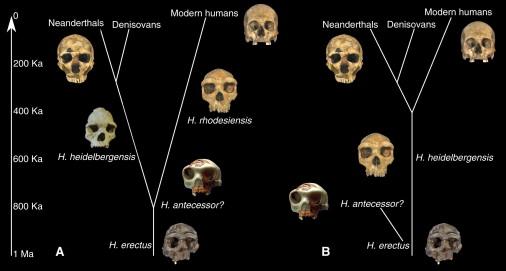
 The first archeologists to find strange stone artifacts on Naxos were French researchers working on the Greek island in 1981. Naxos, the largest in a cluster known as the Cyclades that dot the Aegean Sea, is rich in the type of archeology many would recognize from classical exhibits in museums: 5,000-year-old, beautifully proportioned white marble figurines; 3,000-year-old, strikingly patterned pottery vessels.  Archeologists have long believed that the first people to colonize the region were early farmers who arrived by boat approximately 9,000 years ago. Only humans who had made the leap from a hunter-gatherer subsistence to organized agriculture — a major revolution in the history of our species, one that saw a lurch forward in technological and social complexity — could have accomplished the sea crossing. But the stone tools on Naxos appeared to be hewn by Paleolithic people — much more ancient humans, perhaps not members of our species at all.  Since 2013, Carter has co-directed a new round of investigations on Naxos. He and a handful of others working in the region have begun to furnish evidence that humans reached the islands of the Aegean Sea 250,000 years ago and maybe earlier. If those dates are confirmed, it means the first people there were Neanderthals, their probable ancestors, Homo heidelbergensis or maybe even Homo erectus. In recent years, many of these behavioural firewalls have crumbled. Archeologists have found indications, some still disputed, that Neanderthals carved cave symbols, painted their bodies with pigment, created musical instruments and jewelry, and intentionally buried their dead — all practices thought to be exclusive to us. Most stunningly, scientists have shown that living people with European or Asian ancestry carry a small amount of Neanderthal DNA, evidence of prehistoric interbreeding. |
|
|
|
Post by Admin on Dec 26, 2017 18:57:30 GMT
 Around 600 kya, Europe was invaded by large-brained hominins using Acheulean stone tools (1, 2). They were probably African immigrants, because similar fossils and tools occur earlier in Africa. They have been called archaic Homo sapiens, Homo heidelbergensis, and early Neanderthals, yet they remain mysterious. They may have been ancestors of Neanderthals and modern humans (3), or ancestors of Neanderthals only (4, 5), or an evolutionary dead end. According to this last hypothesis, they were replaced later in the Middle Pleistocene by a wave of African immigrants that separated Neanderthals from modern humans and introduced the Levallois stone tool tradition to Europe (6, 7). To address this controversy, we introduce a statistical method and use it to study genetic data of Africans, Eurasians, Neanderthals, and Denisovans. Our method extends an idea introduced by Reich et al. (8, 9). Their “ABBA-BABA” statistics infer admixture from the frequency with which derived alleles are shared by pairs of samples. As we have shown (10), these estimators have large biases when populations receive gene flow from more than one source. The magnitudes of these biases depend on the sizes and separation times of ancestral populations. Our method avoids bias by estimating these parameters simultaneously. To accomplish this, our method uses an expanded dataset. ABBA-BABA statistics summarize allele sharing by pairs of samples. We extend this approach to include larger subsets, such as trios of samples, and to use all available subsets. This opens a rich and heretofore unused window into population history.  Fig. 1. (A) Population tree representing an African population, XX; a Eurasian population, YY; Neanderthals, NN; and Denisovans, DD. The model involves admixture, mNmN; time parameters, TiTi; and population sizes, NiNi. (B) Population tree with embedded gene tree. A mutation on the solid red branch would generate site pattern ynyn (shown in red at the base of the tree). One on the solid blue branch would generate yndynd. Mutations on the dashed black branches would be ignored. “0” and “1” represent the ancestral and derived alleles. Although our method can accommodate complex models, we work here with a four-population model of history (Fig. 1A), which has broad empirical support (11, 12). In this model, Neanderthals (NN) contribute genes to Eurasians (YY) but not to Africans (XX). The model allows no gene flow from Denisovans (DD), for reasons explained below. Combinations of uppercase letters, such as NDND, refer to the population ancestral to NN and DD. Lowercase letters, such as nn and dd, refer to individual haploid genomes sampled from these populations. The gene tree describes how genes coalesce within the tree of populations. Fig. 1B illustrates one of many possible gene trees. Although closely linked nucleotide sites tend to share the same gene tree, this is not the case for sites farther apart on the chromosome, and any set of autosomal sequence data will encompass a multitude of gene trees. The gene tree determines opportunities for allele sharing among samples. For example, a mutation on the solid red branch in Fig. 1B would be present in yy and nn but absent in xx and dd. We refer to this as the “ynyn site pattern.” Similarly, a mutation on the solid blue branch would generate site pattern yndynd. In a four-population model, there are 10 polymorphic site patterns, excluding singletons. We can tabulate their frequencies in sequence data and calculate their probabilities given particular population histories. Our program, legofit (described in Section S1), estimates parameters by fitting observed to expected frequencies. Whereas ABBA-BABA statistics use only 2 site patterns (“ABBA” and “BABA”), legofit uses all 10. This allows it to estimate additional parameters and avoid the biases discussed above. |
|
|
|
Post by Admin on Dec 28, 2017 18:51:07 GMT
 We studied site-pattern frequencies in four populations at a time: an African population (XX), a Eurasian populaton (YY), Neanderthals (NN), and Denisovans (DD). We use the high-coverage Altai Neanderthal (14) and Denisovan (12) genomes. The modern samples are from Phase I of the 1,000-Genomes Project (15). We study two African populations, the Luhuya (LWK) of East Africa and the Yoruba (YRI) of West Africa. We also study populations from the eastern and western extremes of Eurasia: Europeans (CEU) and northern Chinese (CHB). To identify different analyses, we use abbreviations such as “LWK.CHB,” which means that the African population (XX) is LWK and the Eurasian population (YY) is CHB. We exclude several populations of great interest—Melanesians, the San, and Pygmies—because they would require a different model of history than that in Fig. 1. One set of 10 site-pattern frequencies is shown in Fig. 2A. About 30% of the nucleotide sites in these data exhibit the xyxy site pattern; another 20% exhibit ndnd. Pattern xyxy is common because xx and yy are samples from closely related populations and therefore tend to share ancestry. Mutations in these shared ancestors generate the xyxy site pattern. Shared ancestry also explains the elevated frequency of ndnd.  Fig. 2. (A) Open circles show relative frequencies (horizontal axis) of nucleotide sites exhibiting each site pattern (vertical axis) in four populations: XX, YRI; YY, CEU; NN, Neanderthal; and DD, Denisovan. (B) Expanded view of four site-pattern frequencies, showing 95% confidence intervals, estimated by moving-blocks bootstrap, with 1,000 polymorphic nucleotide sites per block (13). As noted above, our model of history (Fig. 1A) excludes gene flow from Denisovans into Eurasians. This is not a limitation of our method; it is motivated by the structure of the datasets under study. To see why, consider Fig. 2B. Note first that ynyn is more common than xnxn—Neanderthals share more derived alleles with Europeans than with Africans. This suggests gene flow from Neanderthals into Europeans (9). More surprisingly, xdxd is more common than ydyd. The same pattern appears in all four combinations (YRI.CEU, YRI.CHB, LWK.CEU, and LWK.CHB) of African and Eurasian populations in our analysis. This pattern suggests gene flow from Denisovans into Africans, a possibility that we consider in Section S3. It also precludes any estimate of gene flow from Denisovans into Eurasians. For this reason, our base model includes no such term. The analysis proceeds in two stages: one to discover dependencies among parameters and a second one imposing constraints to cope with these dependencies. In stage 1, we fit an unconstrained model to the observed data and also to 50 bootstrap replicates. With the data in Fig. 2A, stage 1 revealed strong dependencies among several parameters (Fig. S1). For example, there is a positive relationship between mNmN, the admixture fraction, and 2NN2NN, the Neanderthal population size (Fig. 3). This relationship makes sense: If the Neanderthal population were large, then most introgressing Neanderthal genes would be distantly related to the Altai Neanderthal fossil. It would therefore take more admixture to produce a given effect on the ynyn site pattern. On the other hand, if the Neanderthal population were small, a little admixture would have a larger effect. |
|
|
|
Post by Admin on Dec 29, 2017 19:02:30 GMT
 Fig. 3. Covariation of estimates of mNmN and 2NN2NN across bootstrap replicates. Data are as in Fig. 2. All four analyses—YRI.CEU, YRI.CHB, LWK.CEU, and LWK.CHB—yield similar results. Estimates of Neanderthal admixture (mNmN) and Neanderthal–Denisovan separation time (TNDTND) appear in Fig. 4. The admixture estimates are 1–3%, in broad agreement with previous results. Our results do not, however, support the view that East Asians carry more Neanderthal DNA than Europeans (12, 14, 17⇓⇓⇓–21). This view may be an artifact of ascertainment bias (17) or of the biases documented by Rogers and Bohlender (10). On the other hand, the East Asian excess may be real, but hidden by the broad confidence intervals surrounding our estimates of mNmN.  Fig. 4. Estimates of Neanderthal admixture (mNmN) and the Neanderthal–Denisovan separation time (TNDTND). The vertical line (Lower) shows TXYNDTXYND. Horizontal lines show 95% confidence intervals based on 50 moving-blocks bootstrap replicates. All point estimates and confidence intervals are based on stage 2 of the analysis. All estimates of TNDTND, the separation time of Neanderthals and Denisovans, are close to 25,600 generations ago—only about 300 generations after the separation of archaics from moderns. Furthermore, this separation time is estimated with high confidence, judging from the narrow confidence intervals in Fig. 4, Lower. During the interval between the two separation events, the ancestral archaic population was apparently very small. Our point estimates of 2NND2NND range from about 100 to about 1,000, with narrow confidence intervals. Following the Neanderthal–Denisovan separation, our results imply a relatively large Neanderthal population, with 2N2N in the tens of thousands. Fig. S3 graphs the history of effective population size of Neanderthals, moderns, and their ancestors, as implied by the YRI.CEU analysis. |
|
|
|
Post by Admin on Dec 31, 2017 19:00:43 GMT
 Fig. S3. History of effective size of Neanderthals (solid red lines), moderns (dashed blue lines), and their ancestors, based on estimates in Figs. 4 and 5 for the YRI.CEU analysis. These simulations also show that estimates of mNmN and 2NN2NN are not as well behaved as those of the other parameters. They exhibit broad confidence intervals in real data (Figs. 4 and 5). In simulations (Fig. 6), they exhibit broad sampling distributions and bias. Presumably this reflects the association seen in Fig. 3. It is difficult to choose between parameter values that lie along the regression line. Our base model (Fig. 1A) omits several forms of gene flow that are known or suspected, and these omissions may have introduced bias. We therefore fitted four alternative models, as described in Section S3. None of these explains the surprising features of our estimates. We have found no way to explain these features as artifacts of a misspecified model.  Fig. 5. Population size estimates. All point estimates are based on stage 2 of the analysis. Confidence interval for 2NXYND2NXYND is based on stage 2; other intervals are based on stage 1. Our estimate of the Neanderthal–Denisovan separation time is surprisingly old. The most recent whole-genome estimate of this parameter is 381 kya (ref. 14, table S12.2), which corresponds to 502 kya or 17,318 generations under our molecular clock. To determine the cause of this inconsistency, we fitted a model in which TNDTND is fixed at 17,318 generations. The red crosses in Fig. 7 show the difference between fitted and observed site-pattern frequencies under this constrained model. The constrained model predicts too much ndnd but too little xndxnd and yndynd. The predicted points lie well outside the confidence intervals. This, along with the smaller discrepancies seen elsewhere in Fig. 7, refutes the hypothesis that Neanderthals and Denisovans separated as recently as 17,318 generations ago.  |
|












