Genetic data suggest that modern European ancestry represents a mosaic of ancestral contributions from multiple waves of prehistoric migration events. Recent studies of genomic variation in prehistoric human remains have demonstrated that two mass migration events are particularly important to understanding European prehistory: the Neolithic spread of agriculture from Anatolia starting ~9,000 years ago, and migration from the Pontic-Caspian Steppe ~5,000 years ago (1-6). These migrations are coincident with large social, cultural, and linguistic changes, and each has been inferred to replace more than half the gene pool of resident Central Europeans during those times. During such events, males and females often experience different demographic histories owing to cultural factors such as norms regarding inheritance and the residence locations of families in relation to parental residence, social hierarchy, sex-biased admixture, and inbreeding avoidance (7-11). Empirical evidence suggests that sex-specific differences in migration and admixture have shaped patterns of human genomic variation worldwide, with notable examples occurring in Africa, Austronesia, Central Asia, and the Americas (12-15). These sex-specific behaviors leave signatures in the patterns of variation in genetic material that is differentially inherited between males and females in a population. Therefore, contrasting patterns of genetic variation for differentially inherited genetic material can be informative about past sociocultural and demographic events (7-11,16).
The spread of chariots: 2000BC onward
Analyses of the maternally inherited mitochondrial DNA (mtDNA) and the paternally inherited Y chromosome have lent differential support for the hypothesis that the Neolithic spread of agriculture from Anatolia occurred through a large population migration, rather than a spread of technology (17-21). In general, studies of Y-chromosomal data more than mtDNA have supported Anatolian migration, which has been interpreted as evidence for male-biased migration of the population that introduced farming. This hypothesis of male-biased migration of farming populations is consistent with ethnographic studies showing a higher frequency of patrilocality in farming than hunter-gatherer populations, as an inheritance model through the paternal lineage would favor the persistence of farming-associated Y chromosomes with more flexibility for the source population of female mates. Isotopic studies from Neolithic European archeological sites suggest more female than male migration on a local scale, supporting the shift to patrilocality in the region (9,22). Based on archeological and modern-day genetic data, the later migration from the PonticCaspian Steppe has also been hypothesized to be male-biased (23-25). Populations in the region, such as the Yamnaya or Pit Grave culture, are thought to have strong male-biased hierarchy, as inferred by overrepresentation of male burials, male deities, and kinship terms (26). The region is a putative origin for the domesticated horse in Europe, and the culture is known for its use of horse-driven chariots, a potential male-biased mechanism of dispersal into central Europe (26). Combining recent analytical advances in the understanding of admixture on the autosomes and the sex-specifically inherited X chromosome with technological advances that have generated genome-wide data from many ancient samples now makes it possible to consider the contrasting male and female genetic histories of prehistoric Europe. We test the hypotheses that migrations from Anatolia during the Neolithic Transition and from the Pontic Steppe during the late Neolithic/Bronze Age period were male-biased.
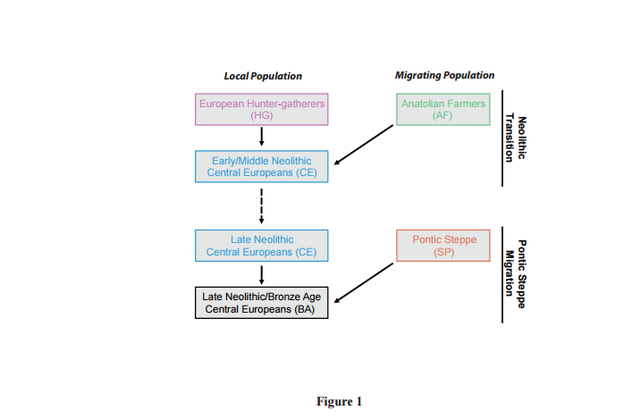
Figure 1
Figure 1 provides a schematic of the population admixture events that have previously been inferred (1-6). Previous studies have inferred the relationship between populations, but did not consider a population history model. We compare genetic differentiation of the autosomes and the X chromosome between the migrating and admixed populations for each migration event: Anatolian farmers (AF) to early Neolithic Central Europeans (CE) and Pontic Steppe pastoralists (SP) to late Neolithic and Bronze Age Central Europeans (BA).
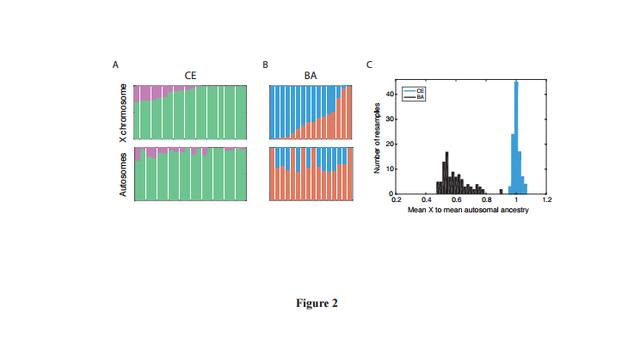
Figure 2
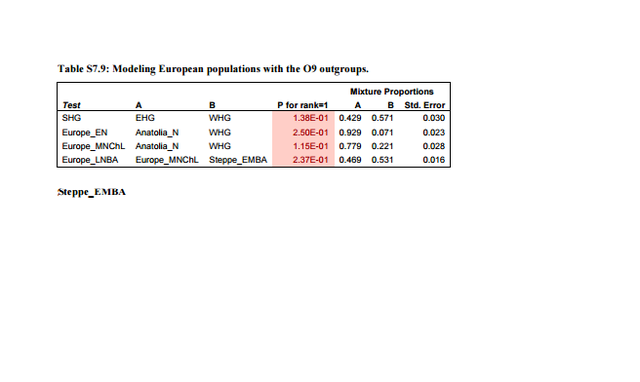
Under a demographic model with equal male and female effective sizes, Q is expected to be ¾, as there are three X chromosome for every four autosomes in the population. Deviations from ¾ would therefore show sex-biased effective population sizes, which indicate different population histories for males and females. Comparing AF and CE populations for the Neolithic transition, X and autosomal differentiation is similar to that expected for a non sex-biased process (Table 1). In contrast, there is high relative differentiation on the X chromosome between SP and BA populations (Q = 0.237, Table 1), indicating strong male bias during the Pontic steppe migration.

Figure 3
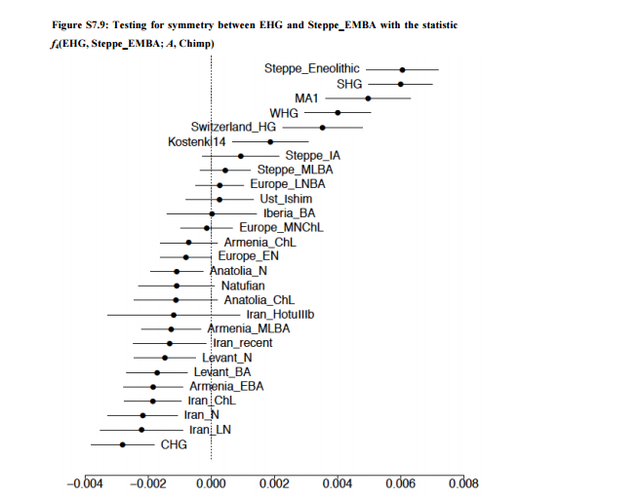
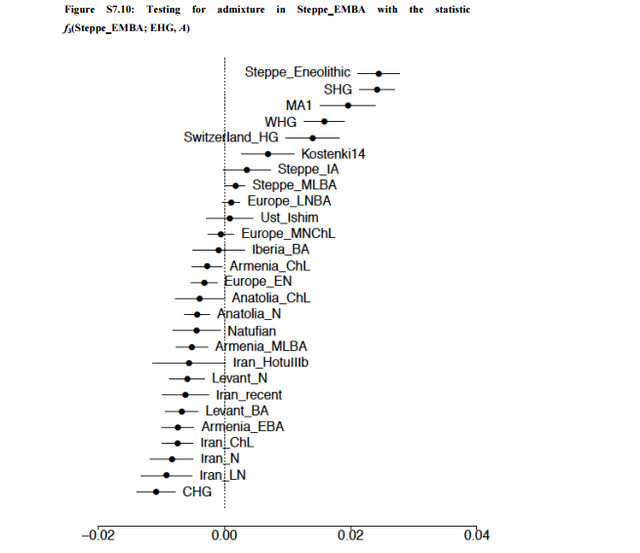
We next considered female and male migration histories during the late Neolithic/Bronze Age migration from the Pontic-Caspian Steppe (Fig. 1). In contrast with the early Neolithic expansion from Anatolia, we find a strikingly lower distribution of SP ancestry on the X chromosome than the autosomes, suggesting extreme male-biased migration from SP during the Late Neolithic/Bronze Age migration from the Pontic-Caspian Steppe. Using a similar approach as that employed for the early Neolithic migration event, the ratio of mean X-chromosomal SP ancestry to mean autosomal SP ancestry in late Neolithic and Bronze Age Europeans (BA) is 0.366 0.618 = 0.592. The ratio of mean X CE ancestry to mean autosomal CE ancestry in the BA population is 0.634 0.382 = 1.66. Of 16 admixed BA individuals, 12 have more SP ancestry on the autosomes than the X chromosome (binomial test, p = 0.038). Similarly, the distribution of p-values of the Wilcoxon sign-rank test comparing the estimated X-chromosomal ancestries to the autosomal ancestries in each of 100 resamples of autosomal SNPs is highly skewed toward zero, with a median of p = 0.02 (Materials & Methods, Fig. S2). To interpret the values of sex-specific admixture that can produce the observed ratio of X-to-autosomal SP ancestry of about 0.6, we considered four models for the admixture process. The first is a single admixture event, in which an SP population quickly mixes with central European farmers, with no further migration from either population to the admixed BA population. Under this model, however, the level of sex bias is too high to have been produced by a single admixture event; no solution for the female and male migration rates exists within the possible admixture contribution range from 0 to 1 (Materials and Methods).
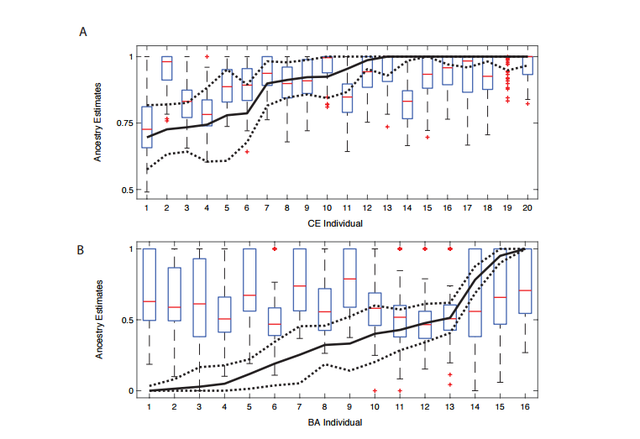
Figure S1: Resampling SNPs to estimate autosomal ancestry.
In other words, in a pulse migration and admixture scenario in a single generation, even a male-only migration event is not extreme enough to generate the observed extreme X-to-autosome bias in the data. Ongoing male migration from the steppe over multiple generations is therefore required to explain observed patterns of X and autosomal ancestry. We therefore considered a model of constant contributions over time from the SP population and early Neolithic farmers (CE). We follow the method of (16), comparing expected X and autosomal ancestry (16, eqs. 19,20; and 30, eq. 31) to observed ancestry in our data over a grid of possible parameter values. We present results from the 0.1% of parameter sets closest to observed data using a Euclidean distance between model-based and observed population mean ancestries on the X and autosomes (Materials and Methods). Other cutoffs (0.5, 1, 5%) showed similar trends. Figure S3 plots the range of sex-specific contributions from the SP and CE populations that produce estimates close to those observed in the BA population. Males from the steppe and central European females show substantial ongoing migration, with continuing admixture rates of almost ½. That is, almost half of the male parents in each generation of BA individuals are new migrants from the SP population. Females from the steppe and early Neolithic European males, however, are estimated to have contributed negligibly to the BA population.
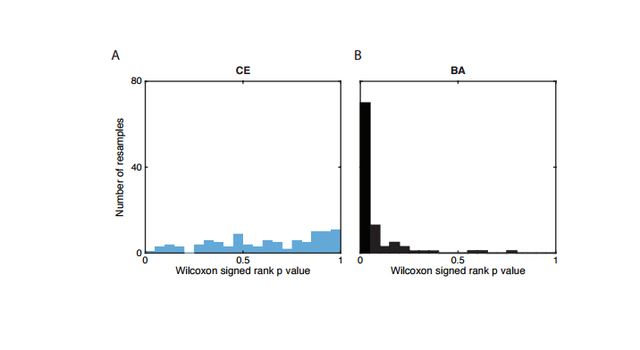
Figure S2: Histogram of p values for the Wilcoxon sign-rank test.
Figure 3B plots the proportional contribution of males from each source population, with a median of about 94% of SP ancestry in the BA population coming from male SP migrants, and all local CE ancestry originating in CE females. This result corresponds to approximately 14 male migrants for every female migrant from the steppe contributing to the ancestry of the BA population. Considering the smallest 0.5%, 1% and 5% of Euclidean distances instead, this ratio is about 8.5, 7.5, and 5.1, respectively, males per female migrating from the steppe. The signature of X-chromosomal to autosomal ancestry is driven by the last few generations of admixture. Testing other models of time-dependent admixture, with the contributions from one or both of the source populations increasing or decreasing over time, we find that the data fit model-based estimates approximately equally well when the admixture contributions at the last few generations are similar to those estimated from a constant admixture model (Materials and Methods).
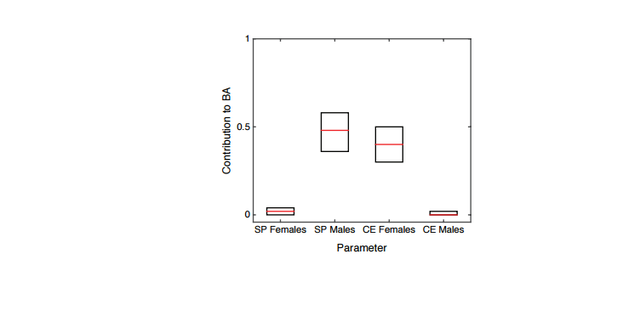
Figure S3: Estimated sex-specific contributions from Pontic Steppe (SP) and early Central Europeans (CE)
to LNBA Europeans (BA) .
The signal of male-biased contributions from SP and to BA over time is consistent with an admixture scenario in which a massive male-biased migration from the steppe initially looks to local European farmer females for wives, and with a paternal mode of inheritance, the BA population disproportionately absorbs females from local ‘unadmixed’ farmers. Admixture from the steppe population continues over time, though mainly men migrate, perhaps expanding using the male-dominated modes of horses and chariots (23,26). Overall, the model-based ancestry results show remarkable similarity to our original comparisons of relative genetic drift on the X versus autosomes using a measure of genetic differentiation (Table 1). Combining observations from both migrations, a picture of sexspecific migrations in central European prehistory emerges (Fig. 3C). Owing to the large ancestry contribution and lack of sex-biased admixture, the massive cultural change that accompanied the shift to agriculture is consistent with a large-scale migration of an entire subset of a population, perhaps families, and a slower rate of spread. Minimal differences in sex-specific migration and the high overall AF ancestry in CE individuals support this scenario. This result suggests that the residence and descent rules were not determining factors in sex-specific migration, despite the probable patrilocality of the migrating AF population (9,22).
The lack of sex bias is in fact consistent with previous indications of sex bias during the Neolithic based on mtDNA diversity. Earlier work focused on measures of diversity rather than ancestry, which will track the effective population size more than admixture. Therefore, earlier single-locus studies are likely seeing the signal of patrilocality rather than the migration process from Anatolia (19). In contrast, our results, combined with the archeological evidence, suggest that the rapid migration from the Pontic Steppe was strongly male-biased, potentially via newly domesticated horses in multiple waves (23,24,26). Such differences in sex-specific migration patterns are suggestive of fundamentally different types of interactions between invading and local populations during the two migration events. Our results demonstrate the power for inferring important processes in human prehistory by analyzing the X chromosome and the autosomes jointly.