|
|
Post by Admin on Nov 25, 2018 20:36:19 GMT
 Slon et al. (2018) analyzed six individuals from Denisova Cave. Denisova 11 was born between a Neanderthal mother and a Denisovan father, who also had some Neanderthal ancestry. It was already known that Denisovans and Neanderthals interbred in an ancient cave in Siberia’s Altai mountains, where they coexisted for tens of thousands of years. The genome of Denosova 11 shares derived alleles with the Altai Neanderthal genome in 12.4% of cases and those present in the Vindija genome in 19.6% of cases, which shows that Denisova 11 was more closely related to Neanderthals found in Vindija. Denisovans share some of their history with Neanderthals before the gene flow from Neanderthals into modern humans occurred and the genome of Denisova 11 provides evidence for genetic admixture between Neanderthals and Denisovans on at least two occasions. But their zones of overlap were restricted in space ant time as Neanderthals largely inhabited in Western Eurasia and Denisovans lived in Eastern Eurasia, especially in Melanesia. As a result, the Papuans and Aboriginal Australians have much more Denisova ancestry (5-6%) compared to Han Chinese (0.2%). 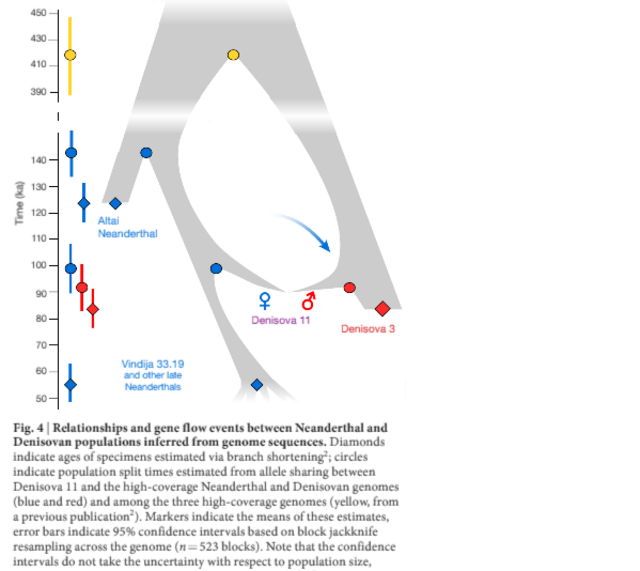 Yes, they had found a human of some kind, but which species? ZooMs can tell only if a bone comes from a member of the hominidae family, which includes great apes and humans, including Homo sapiens, Neanderthals and Denisovans. It cannot differentiate within this group. “There have never been great apes around Denisova so that meant we had to have found a piece of a human,” says Higham. “But which species?” To find out, the sample was taken to Svante Pääbo at the Max Planck Institute for Evolutionary Anthropology in Leipzig, whose team had sequenced the first Denisovan genome in 2010. Initial analysis showed that the bone was more than 50,000 years old and from a person who had been 13 or older when they died. Then the Leipzig team – led by Pääbo’s student Viviane Slon – began more detailed genetic analysis and made a startling discovery. Exactly half the sample consisted of Neanderthal DNA. The other half was made up of Denisovan DNA. At first, the researchers assumed that the sample was contaminated. “I thought they must have screwed up something,” says Pääbo.  Denisova 11 (Denny) bone found as part of the Palaeochron Project and my MSc research. But re-testing confirmed the finding: the Oxford team had discovered the 90,000-year-old remains of a hybrid daughter of a Neanderthal mother and a Denisovan father. She was nicknamed Denny. “If you had asked me beforehand, I would have said we will never find this, it is like finding a needle in a haystack,” Pääbo told Nature. To discover a first-generation person of mixed ancestry was extraordinary. But there were more revelations to come. Further detailed studies of the genes of Denny’s Denisovan father were found to contain fragments of Neanderthal DNA. These indicated that interbreeding between the two species had also occurred at an earlier time.  |
|
|
|
Post by Admin on Nov 27, 2018 18:05:33 GMT
 At first sight, Denny’s remarkable ancestry suggests that Neanderthals and Denisovans must have mated with each other regularly. But Douka counsels caution. “The DNA of Neanderthals and Denisovans are distinct. We can easily tell them apart. That argues against frequent interbreeding. Otherwise they would have ended up with the same DNA.” Past studies have provided clear evidence that Denisovans and modern humans interbred, and also that Neanderthals and modern humans mated with each. Now, thanks to ZooMs – which has since been used to pinpoint other ancient human remains – there is dramatic evidence of Denisovan and Neanderthal intermingling. But why at Denisova? One suggestion is that the cave represents a border outpost for both species, one that was situated at the very eastern edge of the range of the Neanderthals, who were primarily a European species, and at the very western tip of the homelands of the Denisovans, who were an eastern species. Occasionally members from both groups would have reached the cave at the same time – with amorous consequences.  It is an idea borne out by detailed studies of Denny’s Neanderthal mother. Her genes show a particularly close affinity with Neanderthals who lived in Croatia, suggesting that the immediate predecessors of Denny’s mother may have been part of a group who slowly migrated east from Europe towards Denisova – where she encountered Denny’s father at the outer edges of each other’s homelands. It is an intriguing picture, though much more information is required to confirm it. Scientists have no direct evidence that the Denisovans’ homeland range was primarily to the east of the cave, although the fact that their genes have been detected in the DNA of populations in Australia, New Guinea and other parts of Oceania, provides support for this idea and suggests future searches for sites should be focused on eastern Russia, China and south-east Asia.  A great deal more needs to be learned about the Denisovans, says Higham: “What was their distribution? What is the earliest evidence for their emergence from the common ancestor they shared with Neanderthals 500,000 years ago? If we could get a bone or two from other sites, that would be tremendously helpful.” One possible source of fossils could include remains of ancient humans that were placed in museums in Asia decades ago. These could be wrongly labelled, and could be Denisovans, researchers suggest. Unfortunately, it has proved difficult to get hold of these specimens for sampling. For their part, Douka and Higham are planning on a number of approaches. One of these will be to collaborate with researchers in Chinese laboratories, teaching them how to use ZooMs, and how to use the technology to uncover more Denisovan sites. ZooMs is going to be crucial to this project,” says Douka. “We have shown that it is a powerful tool for pinpointing human fossils. That makes it ideal for tracking down Denisovans.” |
|
|
|
Post by Admin on Nov 28, 2018 18:02:23 GMT
 Fig. 1: Location of Neanderthals, Denisovans and ancient modern humans dated to approximately 40 ka or earlier. Denisova 11 could have had approximately equal amounts of Neanderthal and Denisovan ancestry because she belonged to a pop-ulation with mixed Neanderthal and Denisovan ancestry, or because her parents were each from one of these two groups. To determine which of these two scenarios fits the data best, we considered sites at which the genomes of the Altai Neanderthal and Denisova 3 carry a transversion difference in a homozygous form. At each of these sites, we recorded the alleles carried by two randomly drawn DNA fragments from Denisova 11. Note that in 50% of cases, both fragments will come from the same chromosome, making 50% of heterozygous sites appear homozygous. As a consequence, the expected proportion of apparent heterozygous sites is 50% for a first-generation (F1) offspring, whereas it is 25% in a population at Hardy–Weinberg equilibrium with mixed ancestry in equal proportions (Supplementary Information 6). 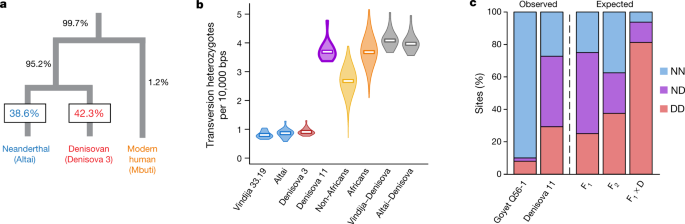 Fig. 2: Denisova 11 has both Neanderthal and Denisovan ancestry. We find that in 43.5% of cases, one fragment from Denisova 11 matches the Neanderthal genome and the other matches the Denisovan genome, whereas in 27.3% and 29.2% of cases both fragments match the state seen in the Neanderthal or the Denisovan genome, respectively (Fig. 2c). For comparison, when a low-coverage Neanderthal genome (‘Goyet Q56-1’)7 is analysed in the same way, the two fragments match different states in 2.1% of cases, while they both match the Neanderthal state in 90.3% of cases and the Denisovan state in 7.5% of cases (Fig. 2c). Obviously, the Altai Neanderthal and Denisova 3 are unlikely to be identical to the genomes of the individuals that contributed ancestry to Denisova 11. To take this into account, we used coalescent simulations to estimate the expected proportions of DNA fragments matching a Neanderthal or a Denisovan genome in populations with demographic histories similar to those of the Altai Neanderthal and Denisova 3 (Supplementary Information 6). The proportion of cases in which one of the two DNA fragments sampled from Denisova 11 matches the Neanderthal state and the other the Denisovan state fits the expecta-tion for an F1 Neanderthal–Denisovan offspring, but not an offspring of two F1 individuals, an offspring of an F1 parent and a Neanderthal or a Denisovan parent, nor an individual from a population of mixed ancestry at Hardy–Weinberg equilibrium (Extended Data Fig. 2 and Supplementary Information 6). |
|
|
|
Post by Admin on Nov 29, 2018 18:06:54 GMT
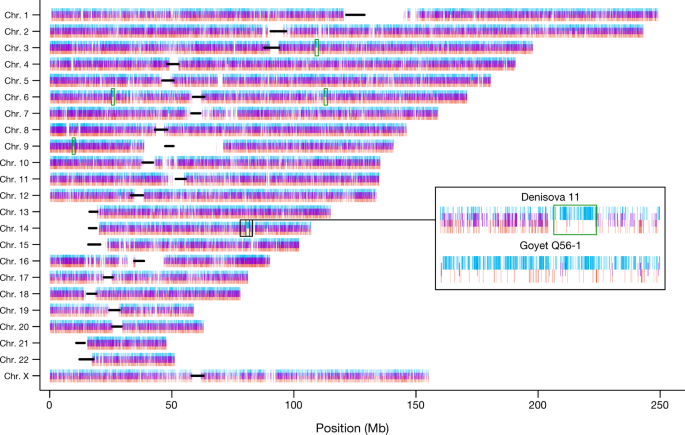 Fig. 3: Distribution of Neanderthal-like and Denisovan-like alleles across the Denisova 11 genome. We conclude that Denisova 11 did not originate from a population carrying equal proportions of Neanderthal and Denisovan ancestry. Rather, she was the offspring of a Neanderthal mother, who contributed her mtDNA, and a Denisovan father.We next plotted the distribution of sites across the genome, for which Denisova 11 carries an allele matching the Altai Neanderthal genome and a different allele matching the Denisova 3 genome. Such sites are distributed largely uniformly (Fig. 3), as would be expected for an F1 offspring of Neanderthal and Denisovan parents. To explore the ancestry of the parents of Denisova 11, we looked for regions in the genome that deviate from a pattern consistent with Denisova 11 being an F1 offspring (Extended Data Fig. 3). Using four tests for enrichment of Denisovan or Neanderthal ancestry, we identify at least five approximately 1-Mb long (0.72–0.95 Mb) regions, all of which are homozygous for Neanderthal ancestry. . 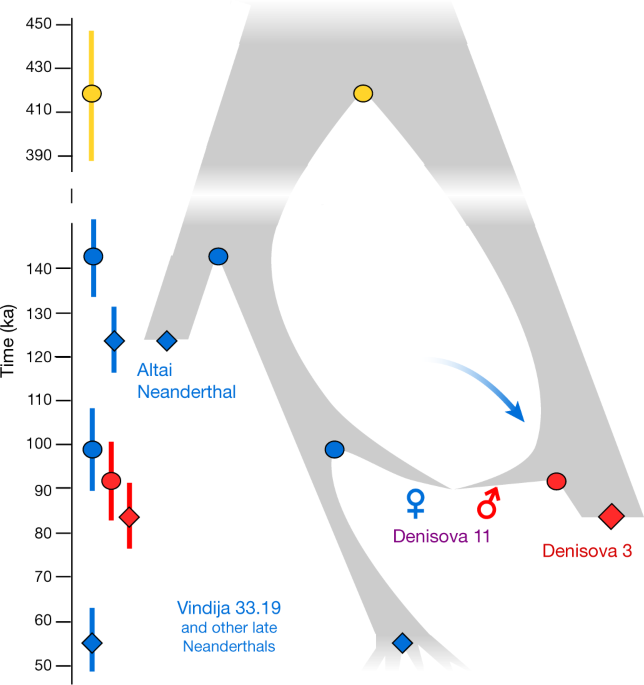 Fig. 4: Relationships and gene flow events between Neanderthal and Denisovan populations inferred from genome sequences. This suggests that the Denisovan father of Denisova 11 had some Neanderthal ancestry. Given conservative estimates of the size and number of these regions, it is likely that there was more than one Neanderthal ancestor in his genealogy, possibly as far back as 300–600 generations before his life-time (Supplementary Information 7). Notably, the heterozygosity in the regions of Neanderthal ancestry in Denisova 11 is higher than in the same regions in the genomes of Vindija 33.19 or the Altai Neanderthal, suggesting that the Neanderthals that contributed to the ancestry of Denisova 11’s father were from a different population than her mother (Supplementary Information 5).To explore how the mother of Denisova 11 was related to the two Neanderthals that have been sequenced to high coverage to date, we evaluated the proportions of fragments from Denisova 11 that match derived alleles from either of these two Neanderthal genomes. Denisova 11 shares derived alleles seen in the Altai Neanderthal genome in 12.4% of cases and those present in the Vindija 33.19 genome in 19.6% of cases, showing that the Neanderthal mother of Denisova 11 came from a population that was more closely related to Vindija 33.19 than to the Altai Neanderthal (Supplementary Information 8). We estimate the population split times of Denisova 11’s Neanderthal mother from the ancestors of the Altai Neanderthal to approximately 20,000 years (20 kyr) before the time when the Altai Neanderthal lived, and her split time from the ancestors of Vindija 33.19 to around 40 kyr before Vindija 33.19. The population split between the Denisovan father of Denisova 11 and Denisova 3 is estimated to approximately 7kyr before the latter individual (Supplementary Information 8). In Fig. 4, we pres-ent a population scenario that is compatible with these observations as well as with the population split times and molecular estimates of the ages of the three high-coverage archaic genomes2. We caution that the age estimates are associated with uncertainties, for example, regarding demography, mutation rates and generation times, and note that addi-tional gene flow events are likely to have affected the population split times. Nevertheless, that a Neanderthal in Siberia who lived approxi-mately 90 ka shared more alleles with Neanderthals who lived at least 20kyr later in Europe2,7 than with an earlier Neanderthal from the same cave8 suggests that eastern Neanderthals spread into Western Europe sometime after 90 ka or that western Neanderthals spread to Siberia before that time and partially replaced the local population. These two non-mutually exclusive hypotheses could be tested by sequencing the genomes of early Neanderthals from Western Europe. Nature. 2018 Sep;561(7721):113-116. |
|
|
|
Post by Admin on Nov 30, 2018 18:08:57 GMT
 Paleolithic occupation of Tibet Human colonization of the high-altitude Tibetan Plateau has generally been thought to have been confined to the past few thousand years of the Holocene. Zhang et al. report an investigation of the Nwya Devu archaeological site in central Tibet, 4600 meters above sea level, with Paleolithic occupation dates of ∼40 thousand to 30 thousand years ago (see the Perspective by Zhang and Dennell). The site has yielded a range of stone tools, indicating the adaptive ability of early modern humans to the harsh environment of the “roof of the world.” The findings also suggest that people from Tibet and Siberia may have interacted at this time. Abstract The Tibetan Plateau is the highest and one of the most demanding environments ever inhabited by humans. We investigated the timing and mechanisms of its initial colonization at the Nwya Devu site, located nearly 4600 meters above sea level. This site, dating from 40,000 to 30,000 years ago, is the highest Paleolithic archaeological site yet identified globally. Nwya Devu has yielded an abundant blade tool assemblage, indicating hitherto-unknown capacities for the survival of modern humans who camped in this environment. This site deepens the history of the peopling of the “roof of the world” and the antiquity of human high-altitude occupations more generally.  An extraordinary theory might explain how humans adapted to life so high above sea level. Most Tibetans carry an unusual stretch of DNA in their genomes, which they seem to have gained when modern humans bred with an ancient group of humans called the Denisovans. The Denisovan DNA seems to help Tibetans cope with the limited oxygen supply at altitude. A 2014 study suggested the Denisovan DNA became more common in the ancestors of today’s Tibetans between about 40,000 and 30,000 years ago, again hinting that modern humans were on the Tibetan Plateau by 30,000 years ago.  But there is an alternative scenario, says John Olsen at the University of Arizona in Tucson, a member of the Nwya Devu excavation team. If modern humans harnessed Denisovan DNA to survive at altitude, perhaps the Denisovans could also survive on the Tibetan Plateau. There are no human remains at Nwya Devu and Olsen says attempts to extract human DNA from the sediment have so far failed. But he says the stone tools might be the handiwork of Denisovans, rather than modern humans. “What we know is that the Denisovans left their homeland in the Altai Mountains of southern Siberia and eventually trekked all the way to Melanesia [the islands northeast of Australia], taking with them their signature genome,” he says. “One logical route for such a migration may have included passage up and over the Tibetan Plateau.” In line with this, Olsen points out that the stone tools at Nwya Devu are similar to those seen in southern Siberia, where we know the Denisovans lived, and not much like those made by Stone Age humans in China. But without fossil or DNA evidence, Olsen says we can only speculate on the identity of the first Tibetans. |
|














