|
|
Post by Admin on Feb 13, 2021 22:54:21 GMT
While the topic of human ancestry is undoubtedly fascinating, EurekAlert! notes that “the meanings of words like ancestor and ancestry are rarely discussed in detail.” That’s where the new study comes in, with a different perspective. A team of experts from the Natural History Museum, The Francis Crick Institute, and the Max Planck Institute for the Science of Human History Jena have presented a new paper titled ‘Origins of modern human ancestry’ in the journal Nature: www.nature.com/articles/s41586-021-03244-5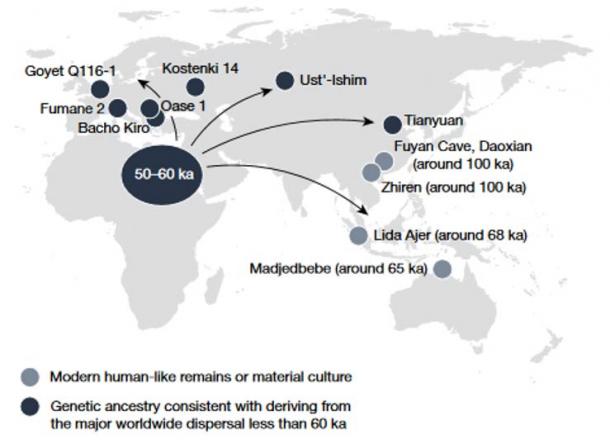 A Single Starting Point Won’t Be Found in the Genetic or Fossil Records The study explores the current understanding about modern human ancestry and how it can be traced back to the distant past, as well as some of the human ancestors found on that timeline. It also asserts that no specific starting point can currently be identified when we’re talking about modern human ancestry. They write: “no specific point in time can currently be identified at which modern human ancestry was confined to a limited birthplace, and that patterns of the first appearance of anatomical or behavioural traits that are used to define Homo sapiens are consistent with a range of evolutionary histories.” Professor Chris Stringer, a co-author in the new study and researcher at the Natural History Museum explained that there just isn’t enough information to work with. He said: Some of our ancestors will have lived in groups or populations that can be identified in the fossil record, whereas very little will be known about others . Over the next decade, growing recognition of our complex origins should expand the geographic focus of paleoanthropological fieldwork to regions previously considered peripheral to our evolution, such as Central and West Africa, the Indian subcontinent and Southeast Asia.  Contrary to what many believe, neither the genetic or fossil record have so far revealed a defined time and place for the origin of our species. Such a point in time, when the majority of our ancestry was found in a small geographic region and the traits we associate with our species appeared, may not have existed. For now, it would be useful to move away from the idea of a single time and place of origin. What Should Researchers Look For Instead? The study identifies three significant phases in human ancestry and major questions which still surround those phases. They suggest that future research should explore these avenues instead of trying to find the elusive starting point of the human story.  The first of the three points of interest is given in the paper as “the worldwide expansion of modern humans between 40 and 60 thousand years ago (ka) and their last known contacts with archaic groups such as Neanderthals and Denisovans.” A second focus “is associated with a broadly construed African origin of modern human diversity between 60 and 300 ka.” Finally, the experts believe there should be more interest in “the complex separation of modern human ancestors from archaic human groups from 0.3 to 1 million years ago.” According to the study co-author Eleanor Scerri from the Pan-African Evolution Research Group at the Max Planck Institute for the Science of Human History, these major questions “concern which mechanisms drove and sustained this human patchwork, with all its diverse ancestral threads, over time and space.” Furthermore Scerri clarified that “Understanding the relationship between fractured habitats and shifting human niches will undoubtedly play a key role in unravelling these questions, clarifying which demographic patterns provide a best fit with the genetic and palaeoanthropological record.” |
|
|
|
Post by Admin on Feb 16, 2021 20:39:16 GMT
 The entangled history of Homo sapiens and Neanderthals in the Levant (the area around the eastern end of the Mediterranean) just got even more complicated. Paleoanthropologists recently identified a tooth from Shukbah Cave, 28km (17.5 miles) northwest of Jerusalem, as a Neanderthal molar. That makes Shukbah the southernmost trace of Neanderthals ever found, and it also links our extinct cousins to a stone tool technology previously considered an exclusive trademark of Homo sapiens. The Levant was one of the first areas hominins reached when they began to expand beyond Africa, and the archaeological record suggests that early expansion happened in a series of waves. At some sites, layers of artifacts show that members of our species lived there for a while before being replaced by Neanderthals, and vice versa. It was a geographical crossroads, and like all such places, its story is dynamic and complex—and it can be hard to piece together from the bits of bone and stone left behind. Often, stone tools are archaeologists’ best clue about who lived at a site and when. There are many ways to shape a piece of flint into something useful like a scraper or a hand ax, and archaeologists recognize different cultures based on subtle differences in those methods and the shape of the resulting tools. One approach to toolmaking, which produces distinctive stone points, is called Nubian Levallois. It’s one of several variations on a general theme of chipping flakes off a prepared stone core to produce a tool. Another variation on that theme is Mousterian technology, which is usually found at Neanderthal sites in western Europe. Nubian Levallois tools tend to turn up at sites from southern Africa to northeastern Africa. Until recently, archaeologists have assumed that Nubian Levallois was a trademark of our species in Africa and the Levant, while Mousterian was a trademark of Neanderthals. But the Neanderthal molar (uncovered by archaeologist Jimbob Blinkhorn of Royal Holloway, University of London and his colleagues) was buried in a layer of sediment alongside a mixture of Mousterian and Nubian Levallois tools. “This is the first time they’ve been found in direct association with Neanderthal fossils, which suggests we can’t make a simple link between this technology and Homo sapiens,” said Blinkhorn. Making a mountain from a molar The lone tooth from Shukbah—a lower first molar—spent most of the last century in the private collection of Sir Arthur Keith. It was eventually donated to the Natural History Museum in London, so archaeologists are only recently getting to take a close look at it. “Broadly, hominin fossils are rare, and so this was a fantastic opportunity to study this find in greater detail and open up wider comparisons on the Neanderthal populations of southwest Asia,” Blinkhorn told Ars. Blinkhorn and his colleagues used computed tomography (CT) scans to measure the internal and external shape and structure of the tooth. They compared those shapes and measurements to other Neanderthal and Homo sapiens molars from southwest Asian sites. In the end, the tooth clearly belonged in a category with the Neanderthal molars. And the Neanderthal in question seems to have been a young child, probably around 9 years old, just getting their first permanent teeth in. The first molar is usually one of the first permanent teeth to grow in, and this one showed hardly any signs of wear, which suggests that it was fairly new. So far, efforts to get ancient DNA from the tooth haven’t succeeded: “A previous team have tried this, and the drill hole is evident on the image of the tooth, but as far as I am aware this was unsuccessful,” Blinkhorn told Ars. 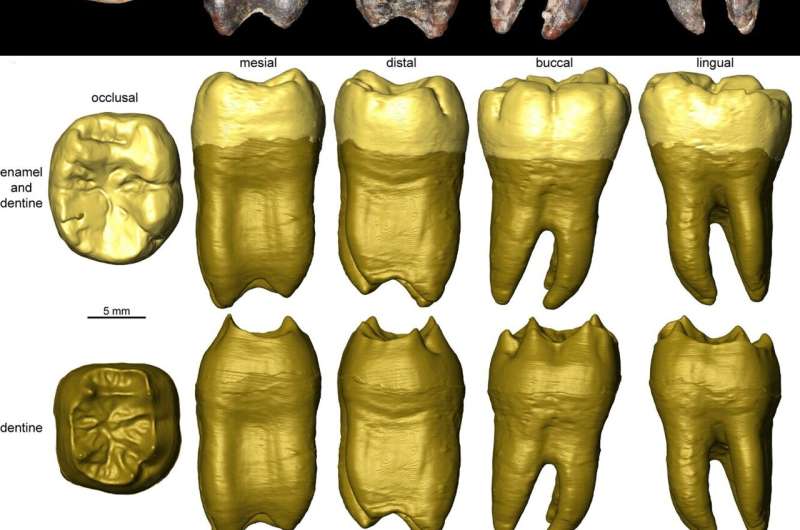 “Illustrations of the stone tool collections from Shukbah hinted at the presence of Nubian Levallois technology, so we revisited the collections to investigate further,” said Blinkhorn. “In the end, we identified many more artifacts produced using the Nubian Levallois method than we had anticipated.” Finding fossils alongside stone tools is relatively rare, but when it happens, it links ancient hominins directly with the things they made and used. Archaeologists rely on those rare links to identify the makers of stone tools at other sites where no fossils remain. Stone tool technologies linked to a particular hominin species or culture help archaeologists track how, where, and when early humans moved through the world. But the Shukbah Cave molar suggests it’s actually not that simple. “This study... issues a timely note of caution that there are no straightforward links between particular hominins and specific stone tool technologies,” said study co-author Simon Blockley, an archaeologist at Royal Holloway, University College of London. Same idea, different times and places Blinkhorn, who specializes in stone tools, told Ars that Neanderthals probably figured out the Nubian Levallois method on their own, separately from groups of H. sapiens who also invented the technology at different times and places. If he’s right, it’s similar to how human cultures around the world have independently arrived at the same solutions for other technological challenges, from pyramids to bows and arrows to fishing.  “Within Africa, there is evidence for multiple, independent innovations of Nubian Levallois technology. Its identification in southern Africa appears disconnected from its appearance in northern/eastern Africa,” Blinkhorn told Ars. “Given the common background in using other Levallois methods, the simplest explanation is that Neanderthals also separately developed Nubian Levallois methods. Other scenarios are also possible, of course, especially given the overlapping and mingling of hominin species in the Levant at the time. As always in archaeology, additional evidence is needed to draw more detailed conclusions. Scientific Reports, 2021 DOI: 10.1038/s41598-001-822576 |
|
|
|
Post by Admin on Feb 25, 2021 4:28:39 GMT
The study seemingly resolves a long-standing scientific debate over our ancestor's ability for brachiation — the ability to swing from tree limbs only using one's arms. Before this ancestor experienced an evolutionary shift toward using hands for tools and legs for walking, they likely knuckle-walked on the ground and glided across canopies. WHAT'S NEW — Research published Wednesday in the journal Science Advances suggests the last common ancestor of hominids — a category of great apes that includes chimpanzees, gorillas, orangutans, and humans — climbed and swung in trees. "Our findings support the view that humans and chimpanzees evolved from an ancestor that had similarities to modern apes in their locomotor adaptation," lead author Thomas C. Prang, an assistant professor at Texas A&M University, tells Inverse. SOME BACKGROUND — Most scientists recognize that the highly dextrous human hand seems to differ in shape and form from the hands primates use to swing from trees. However, this evidence has given rise to a disputed hypothesis: Humans evolved from a quadrupedal ancestor that used all four limbs for movement on the ground, rather than a bipedal ancestor that suspends from trees. Proponents of this hypothesis believe the last common ancestor was more "monkey-like" and less similar to, say, chimpanzees or bonobos. The researchers in this study were skeptical of this idea and wanted to test its merits. HOW THEY DID IT — Researchers used a sample of 400-plus specimens, encompassing both living primates and ancient hominoid fossils. First, researchers analyzed the ancient hand bones of Ardipithecus ramidus, which believers of the disputed hypothesis use to support their idea regarding a quadrupedal last common ancestor. Ardipithecus ramidus is a human ancestor that lived nearly 4.4 million years ago. Our understanding of it is predominantly linked to a partial skeleton found in 2009, nicknamed 'Ardi.' The initial interpretation of this hand suggested the last common ancestors of humans and chimpanzees used a form of locomotion called "above-branch clambering," Prang explains.  He doubts this interpretation for one reason: monkeys and lemurs are the only primates that use above-branch climbing, and their much smaller bodies use external tails to help them with tree climbing — unlike the subject of their study. "The inference of 'above-branch' adaptations in Ardipithecus is somewhat problematic since it's chimpanzee-sized and lacks an external tail [like all apes and humans]," Prang says. To test it, Prang and his colleagues reconstructed the evolution of the hominin hand and how it may have adapted in ancient environments. 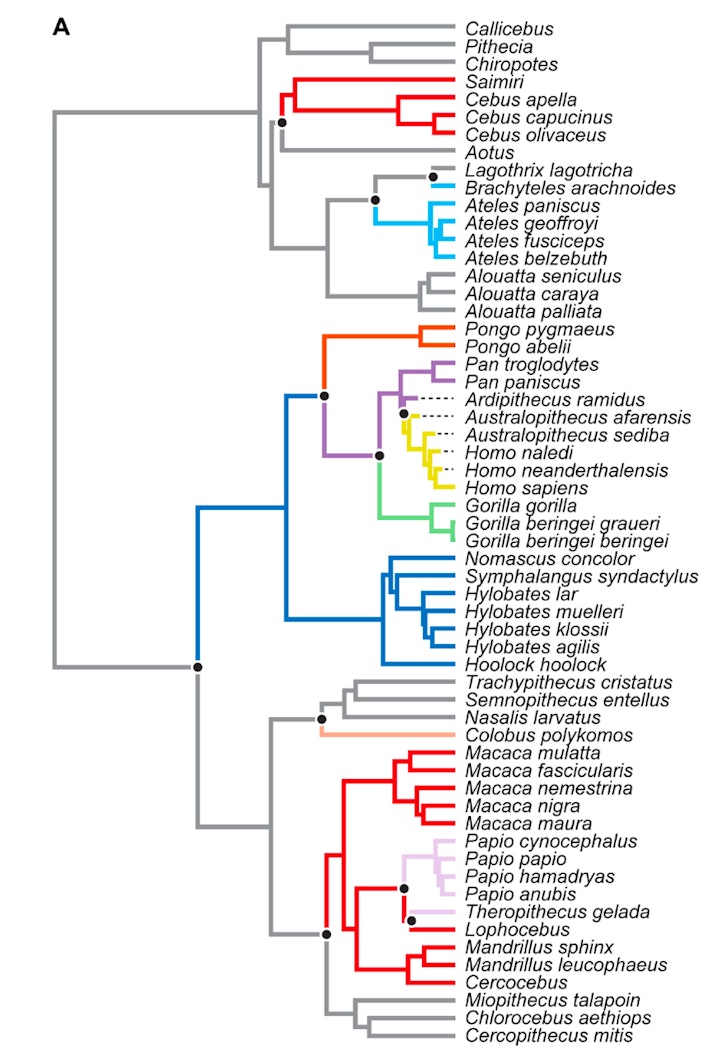 Ar. ramidus had these suspensory traits — which enabled them to swing from tree branches — before a significant evolutionary shift occurred with the lineages of Homo (humans) and Australopithecus, an ancient ancestor of hominins, which includes humans and chimpanzees. "The hand of Ardipithecus suggests that the last common ancestor of humans and chimpanzees was adapted to climbing tree trunks and suspending the body beneath branches," Prang says. The study, in turn, is framed as a debunking of the earlier hypothesis suggesting hominins evolved from an ancestor "with a generalized hand that lacked suspensory adaptations." According to Prang, the study also indicates an important evolutionary step related to the development of tool use. "We show a major evolutionary jump between the hand of Ardipithecus and all later hominins that happens to coincide with the loss of tree climbing adaptations in the foot and the earliest known stone tools and stone tool-cut-marked animal fossils," Prang says. This finding provides support for the idea that Ar. ramidus displayed an early form of bipedalism — or the ability to walk upright on two legs — which helps us understand how human hands and feet evolved. "Our study provides some support for the hypothesis that human hands and feet 'co-evolved,' which previous studies have suggested on the basis of comparisons of patterns of hand/foot trait relationships, and evolutionary simulations, among humans and chimpanzees," Prang says. Alexandros Karakostis, a hand biomechanics expert not affiliated with the study, describes the findings to Inverse as "very intriguing." It provides a robust answer to "a heated debate," Karakostis says — although it's a debate that's likely to continue. "In this context, this new study identifies suspensory adaptations in the 4.4 million-year-old hand remains of Ardipithecus ramidus, suggesting that human hand morphology may have emerged from an evolutionary shift between Ardipithecus and Australopithecus," he says. |
|
|
|
Post by Admin on Feb 25, 2021 20:25:25 GMT
Ardipithecus hand provides evidence that humans and chimpanzees evolved from an ancestor with suspensory adaptations
Thomas C. Prang1,*,
Kristen Ramirez2,3,4,
Mark Grabowski5,6 and
Scott A. Williams2,7,8
Science Advances 24 Feb 2021:
Vol. 7, no. 9, eabf2474
DOI: 10.1126/sciadv.abf2474
Abstract
The morphology and positional behavior of the last common ancestor of humans and chimpanzees are critical for understanding the evolution of bipedalism. Early 20th century anatomical research supported the view that humans evolved from a suspensory ancestor bearing some resemblance to apes. However, the hand of the 4.4-million-year-old hominin Ardipithecus ramidus purportedly provides evidence that the hominin hand was derived from a more generalized form. Here, we use morphometric and phylogenetic comparative methods to show that Ardipithecus retains suspensory adapted hand morphologies shared with chimpanzees and bonobos. We identify an evolutionary shift in hand morphology between Ardipithecus and Australopithecus that renews questions about the coevolution of hominin manipulative capabilities and obligate bipedalism initially proposed by Darwin. Overall, our results suggest that early hominins evolved from an ancestor with a varied positional repertoire including suspension and vertical climbing, directly affecting the viable range of hypotheses for the origin of our lineage.
INTRODUCTION
The morphology and inferred positional behavior of the last common ancestor (LCA) of humans, chimpanzees, and bonobos (hereafter, “LCA”) are critical for understanding the evolution of hominin bipedalism (1–5). Numerous adaptive explanations for bipedalism rely on an understanding of our place in nature. The recognition that humans are closely related to African apes influenced the range of possible explanations for bipedalism by raising questions about the kind of ancestor from which the human bauplan was derived (1). Keith (6) established the hypothesis that human postcranial anatomy was derived from an orthograde ancestor, later interpreted as a “brachiating” ancestor (7), by highlighting the aspects of trunk and limb anatomy shared among humans and other hominoids.
The morphology of the highly dexterous human hand, with its intrinsically elongated first ray (pollex or thumb), shortened metacarpals and nonpollical digits, and hypertrophied thenar muscles, contrasts sharply with that of suspensory adapted anthropoid primates (8–10). The most suspensory hominoids, cercopithecoids, and platyrrhines tend to display a reduced pollex and narrow, elongated nonpollical rays. For example, Pan, Pongo, and Colobus have a markedly reduced thumb, and Ateles and Brachyteles have entirely lost an external thumb (8, 11). In these features, humans were argued to more closely resemble cercopithecoid primates than hominoids (10–12). The recognition that human and suspensory hominoid hands are specialized in seemingly divergent directions influenced the development of an alternative hypothesis in which humans evolved from a quadrupedal, cercopithecoid-like ancestor instead of a suspensory, hominoid-like one (10–12).
Detailed comparative research demonstrated that the hands of humans and cercopithecoids are only superficially similar in morphology (13–15). For example, although humans have an intrinsically elongated thumb compared to most hominoids, the lengths of the nonhuman hominoid pollical metacarpal and phalanges are not reduced relative to their body size (13). Furthermore, humans and other hominoids share an intra-articular meniscus on the ulnar side of the wrist responsible for isolating the ulnar styloid process (14, 16). Humans also share with chimpanzees, bonobos, and gorillas the fusion of the os centrale and scaphoid (1, 13, 15), which increases midcarpal joint stability and decreases joint stress (1). The shared aspects of hand and wrist morphology among humans and nonhuman hominoids, especially the African apes, supported Keith’s hypothesis (6) that humans evolved from a hominoid-like ancestor.
However, later discoveries of Miocene hominoid fossils preserving primitive postcranial morphology relative to extant hominoids suggested that the anatomical correlates of below-branch suspension might have evolved multiple times throughout hominoid evolutionary history (2–4, 17, 18). Few Miocene hominoids have traits that reflect the undisputed use of hominoid-like suspensory locomotion (19), with the potential exception of Hispanopithecus laietanus (18) and Danuvius guggenmosi (20). For example, the Miocene fossil hominoids Sivapithecus and Pierolapithecus have dorsally oriented articular surfaces of their manual proximal phalanges, indicating the habitual use of extended metacarpophalangeal (MCP) joints in palmigrade quadrupedalism (21).
The hand of the early Pliocene hominin partial skeleton ARA-VP-6/500 attributed to Ardipithecus ramidus has been argued to support the hypothesis of a nonsuspensory LCA (2–4, 22). The initial analysis of the ARA-VP-6/500 hand concluded that Ar. ramidus had none of the adaptations associated with forelimb-dominated suspension present among extant hominoids (2–4). Instead, the hand of Ar. ramidus was argued to be reminiscent of more “generalized” (in some ways monkey-like) Miocene hominoids such as Ekembo and Pierolapithecus. If Ar. ramidus did not have a suspensory-adapted hand and instead had a more generalized morphology, then human hand morphology may be less derived than that of chimpanzees and bonobos relative to the LCA (2, 22). In this case, the distinctive pollical to nonpollical ray proportions of modern humans would be considered an exaptation, rather than an adaptation, useful for manipulation and tool use (23, 24) since it originated in the context of Miocene hominoid arboreal quadrupedal locomotion and feeding behavior (22, 25).
Thus, inferences about the positional behavior of fossil hominoids and hominins have contributed to a more recent alternative hypothesis, which proposes that each extant hominoid lineage was independently derived for suspensory posture and locomotion from more generalized ancestors with “cautious climbing” adaptations (2–4). This alternative hypothesis is a refinement of Straus’ earlier hypothesis (10, 11), in that it concedes the phylogenetic position of humans as the sister taxon of chimpanzees and bonobos within the great ape clade, combined with suggestions that cautious climbing, not suspension, explains the locomotor anatomy of hominoids (16, 26).
Here, we test the hypothesis that hominins evolved from an ancestor that lacked adaptations for below-branch suspension using metric data from a large and diverse sample of extant primates and fossil hominins with special emphasis on the hand of Ar. ramidus. We combine standard morphometric analyses with phylogenetic comparative methods that allow us to reconstruct the adaptive landscape of hominin hand evolution, placing fossil taxa within shared selective regimes. Species in a selective regime share a common selective factor, e.g., positional behavior, where regime shifts imply evolution toward new adaptive optima brought on by a change in selection. First, we evaluate the morphometric affinities of the Ar. ramidus hand and show that it is most similar to chimpanzees, bonobos, and orangutans among a sample of 53 anthropoid primate species. Second, we reconstruct the evolution of the hominin hand and show that Ar. ramidus evolved in a selective regime shared with modern chimpanzees and bonobos to the exclusion of later hominins. Overall, our results (i) provide a more refined view of the positional behavior of Ar. ramidus and the Homo-Pan LCA, (ii) resolve a long-standing debate about the role of suspension in the ancestry of humans (2–4, 7, 27), and (iii) imply coevolutionary shifts in hominin hand and foot morphology associated with manipulation and obligate bipedality, respectively (23, 24, 28).
|
|
|
|
Post by Admin on Feb 26, 2021 4:16:06 GMT
RESULTS Morphometric analyses To evaluate alternative evolutionary trajectories for the hominin hand, we conducted a principal components analysis (PCA) on 26 logged geometric mean-standardized measurements of metacarpals and phalanges preserved in the ARA-VP-6/500 fossil [first metacarpal (MC1), fifth metacarpal (MC5), third proximal phalanx (PP3), and third intermediate phalanx (IP3)] on the full comparative sample. Our results show that PCA separates extant anthropoid primates according to known differences in hand morphology (Fig. 1 and fig. S1). Hylobatids, Ateles, Brachyteles, and Homo occupy distinct areas of the morphospace associated with their highly specialized hands related to variation in the proportions of the pollical and nonpollical rays. The first principal component (PC1) accounts for 47% of the variance and is primarily associated with the relative lengths of the phalanges and MC5 combined with MC1 variables (table S1 and figs. S2A and S3A). The second principal component (PC2) accounts for 16% of the variance and is primarily associated with MC1 and MC5 variables (figs. S2B and S3B). The third principal component (PC3) separates hominoids from most other anthropoids with little overlap. PC3 accounts for 13% of the variance and is primarily associated with carpometacarpal joint, MCP joint, and MC1 midshaft dimensions (figs. S2C and S3C).  Fig. 1 PCA on 26 logged geometric mean-standardized variables representing between-species variation in hand shape. Each point is a species mean, except among fossil hominins, and colors represent selective regimes identified by SURFACE. Homo and Australopithecus, gold; Ar. ramidus, P. troglodytes, and P. paniscus, purple; G. gorilla, Gorilla beringei beringei, and Gorilla beringei graueri, light green; Pongo pygmaeus and Pongo abelii, orange; Hylobates, Hoolock, Nomascus, and Symphalangus, blue; Macaca, Mandrillus, Cercocebus, Cebus, and Saimiri, dark red; Papio and Theropithecus, pink; Ateles and Brachyteles, light blue; Colobus, tan; remaining platyrrhines, cercopithecins, and colobines, gray. Note that most cercopithecoids and platyrrhines fall along a spectrum of generalized palmigrady to specialized digitigrady. The Ar. ramidus and Au. afarensis hands fall nearest to a hypothetical evolutionary trajectory from a suspensory, Pan-like ancestor instead of a more generalized, monkey-like ancestor. Purple and gray clouds represent hypothetical uncertainties surrounding suspensory Pan-like and generalized ancestral morphotypes, respectively. Ar. ramidus and Australopithecus afarensis fall along an evolutionary trajectory from a suspensory LCA when examining the first three PCs together (Fig. 1). The placement of Au. afarensis between modern humans and gorillas on PC1 is consistent with a recent resampling analysis of hand proportions (29). While Homo naledi is nearly indistinguishable from modern humans, Australopithecus sediba and Homo neanderthalensis are distributed in different directions around the modern human mean, which is consistent with the results of previous studies (30). A multivariate cluster analysis on Euclidean distances between PC scores (unweighted pair group with arithmetic mean) results in a great ape cluster including Ar. ramidus nearest to Pan and Pongo (fig. S4). All other fossil hominins cluster exclusively with modern humans. To compare the hand of Ar. ramidus (ARA-VP-6/500) to the fossil hominoid H. laietanus (IPS18800), we conducted a second PCA on 17 logged geometric mean-standardized measurements of metacarpals and phalanges preserved across both hand fossils (MC4, PP3, and IP3). PCA on this modified dataset also separates extant anthropoid primates according to known differences in hand morphology but with less separation among groups since the MC1 is excluded (figs. S5 and S6 and table S2). The Ar. ramidus hand (ARA-VP-6/500) falls within the Pan distribution along PC1 and PC2 and within the overlapping distributions of Pan, Lagothrix, and cercopithecoids along PC1 and PC3 (figs. S5A and S6A). The H. laietanus hand (IPS8800) is positioned intermediately between Pongo and papionins, and within the interquartile range of Ateles, along PC2 (figs. S5B and S6B). The Ar. ramidus hand is clearly Pan-like according to the PCA on the modified dataset, whereas the hand of the great ape H. laietanus is positioned intermediately between suspensory taxa (e.g., Pongo and Ateles) and quadrupedal monkeys. Additional details on PCA loadings (table S2) are outlined in the Supplementary Materials. To supplement our initial PCA and to offer an additional perspective on the morphometric affinities of the Ar. ramidus hand, we conducted discriminant function (DFA) and canonical variates analyses (CVA) on 26 logged geometric mean-standardized measurements of metacarpals and phalanges (MC1, MC5, PP3, and IP3). The DFA correctly classifies 97% of individuals using leave-one-out cross-validation (table S3). All fossil hominin hands were classified as “Homo” with a high posterior probability, except the Ar. ramidus hand, which was classified primarily as “Pan” (table S4). Extant hominoid groups are well separated from each other and from cercopithecoids and platyrrhines in the CVA (fig. S7, A and B, and table S5). The Ar. ramidus hand is positioned on the positive end of Canonical Axis 2 (CAN2) within the ranges of Gorilla, Pongo, and hylobatids and outside the ranges of all other groups (fig. S7A). The A.L. 333 and MH2 fossil hands are classified as Homo but are positioned just outside of the range of Homo sapiens along Canonical Axis 1 (CAN1) (fig. S7A). The Ar. ramidus hand falls within the Pan distribution along CAN1 and Canonical Axis 3 (CAN3) (fig. S7B). Additional details on the coefficients of linear discriminants (table S5) can be found in the Supplementary Materials. We examined multivariate variation in MCP and interphalangeal (IP) joint shape using PCA on six logged geometric mean-standardized measurements [MC5 head mediolateral breadth (MC5 HML); MC5 head dorsopalmar depth (MC5 HDP); PP3 trochlea mediolateral breadth (PP3 TML); PP3 trochlea dorsopalmar depth (PP3 TDP); IP3 trochlea mediolateral breadth (IP3 TML); IP3 trochlea dorsopalmar depth (IP3 TDP)] that are linked to the mechanics of suspension (Fig. 2A). In unimanual suspension with the hand positioned in a “hook grip” on a circular horizontal support (9, 31) and with the MCP joint in approximately 90° of flexion (32), the force of body weight (mg) creates an extension moment at the MCP joint with an external moment arm (R) determined by the distance between the weight vector and the support reaction force (SRF). Opposing MCP flexion moments are generated by the isometric force production (FFD) of digital flexors (e.g., m. flexor digitorum profundus and m. flexor digitorum superficialis) and lumbricals with a moment arm length (r) influenced by the sagittal shape profile of the MCP joint (Fig. 2A). This simplified biomechanical model builds on previously published models describing the application of internal and external forces hypothesized to produce phalangeal stress and strain (32, 33). The points of application, positions, spatial orientations, and magnitudes of external and internal force vectors, as well as the moment arm lengths, are hypothetical. Their purpose is to illustrate the biomechanical relevance of variation in MCP and IP joint shape in suspension.  Fig. 2 MCP and interphalangeal joint shape contributes to suspensory performance. (A) Free-body diagram depicting the third ray of a siamang positioned in a hook grip on a horizontal support following (32). The points of application, spatial orientations, and magnitudes of external and internal force vectors, as well as moment arm lengths, are hypothetical. Joint reaction forces are not depicted here for the sake of clarity. SRF, support reaction force (near proximal, middle, and distal phalanges); R, external moment arm; r, internal moment arm; FFD, muscle force vector associated with the flexor digitorum muscles (m. flexor digitorum superficialis and m. flexor digitorum profundus); FFDP, muscle force vector associated with the m. flexor digitorum profundus. Flexor forces applied to the support are the vertical components of FFD and FFDP and the horizontal component of FFD′. (B) Major axis of variance derived from a PCA on six logged geometric mean-standardized measurements (MC5 HML, MC5 HDP, PP3 TML, PP3 TDP, IP3 TML, and IP3 TDP). Positive values of PC1 are associated with decreased MC5 head breadth, increased PP3 TDP, and increased IP3 TDP. The first PC accounts for 62% of the variance, and positive values are associated with decreased MC5 HML, increased PP3 TDP, and increased IP3 TDP (Fig. 2B). Higher values of PC1 therefore reflect increases in morphological measurements hypothetically correlated with the moment arms of the extrinsic digital flexors (e.g., m. flexor digitorum superficialis and m. flexor digitorum profundus). Hylobatids, Pan, Pongo, Gorilla, and atelines (Ateles, Brachyteles, and Lagothrix) fall to the right of papionins, cercopithecins, and colobines along PC1, with some overlap between gorillas and arboreal cercopithecoids and platyrrhines (Fig. 2B). Ar. ramidus falls within the ranges of variation of Pan troglodytes, Gorilla gorilla, and Pongo and above the ranges of variation of all cercopithecoids and platyrrhines. Australopithecus and Homo fall within the range of variation of H. sapiens, which occupies the negative end of PC1 overlapping with arboreal cercopithecoids and platyrrhines. The Ar. ramidus ARA-VP-6/500 hand does not preserve the head of the third metacarpal (MC3), so we necessarily analyzed the articular dimensions of the preserved MC5 head alongside the PP3 and IP3 dimensions. Our biomechanical model depicts the MCP and interphalangeal joints of the third ray (i.e., including the third metacarpal head), but it applies to all nonpollical rays. We examined MC1 traits hypothesized to reflect the ability of modern humans and fossil hominins to generate forceful pollical grips and to reduce stress at the pollical MCP and carpometacarpal joints [i.e., MC1 head breadth, MC1 head depth, MC1 cross-sectional area (product of MC1 MSML and MC1 MSDP), and MC1 base area (product of MC1 BML and MC1 BDP)] associated with stone tool–related behaviors (30, 34). Modern humans have dorsopalmarly deeper (fig. S8A), mediolaterally wider (fig. S8B) MC1 heads combined with large diaphyseal cross-sectional and base areas relative to overall hand size (quantified as the geometric mean of 26 metrics) compared to other taxa (fig. S8, C and D). H. neanderthalensis, H. naledi, Au. sediba, and Au. afarensis tend to be most similar to modern humans in most of the comparisons, whereas Ar. ramidus is consistently Pan-like. However, H. naledi has an exceptionally small MC1 base relative to hand size, as initially noted by Kivell and colleagues (35), and is similar to Ar. ramidus and Pan (fig. S8C). In addition, Au. sediba has an MC1 base area that is intermediate between Homo and Pan relative to hand size. |
|













