|
|
Post by Admin on Oct 3, 2023 19:22:54 GMT
 Fig. 5. Summary of the ancestry-specific IBD sharing. The pie charts represent the proportion of autochthonous southern African-related (i.e., neither Bantu- nor East African–related) (A) and Bantu-related (B) IBD shared on average between each target group and the pools of populations identified in the figure legend. The category “Unknown” represents southern African–related IBD shared with the peripatetic groups from the Angolan Namib. The percentage of southern African (A) and Bantu (B) ancestry within each target group is represented by the total area of the pie chart. (C) and (D) show a UPGMA clustering of all target groups according to their shared southern African– and Bantu-related IBD, respectively. Detailed results for every population pair are shown in fig. S20. Population abbreviations according to (C). In the Angolan Namib, the formerly Kwadi-speaking Kwepe and the other peripatetic groups all share Bantu-related ancestry with the southwestern Bantu pastoralists that surround them (Fig. 5 and figs. S19 and S20). However, their southern African–related ancestry does not match any of the major ancestry components that have previously been described in southern Africa (Fig. 5 and figs. S19 to S21): Despite sharing large amounts of southern African–related IBD segments among themselves, the peripatetics stand out for their lack of IBD sharing with present-day southern African forager groups (Fig. 5 and figs. S20 and S21), suggesting that their southern African–related ancestry resulted from admixture with a deeply divergent unsampled group. The same ancestry is also found in other groups from southwestern Africa, including the Damara from Namibia, but the detected frequencies are much lower than in the Angolan peripatetics (Fig. 5 and figs. S20 and S21). The uniqueness of this previously undetected genetic component [henceforth called Khoisan (KS)–Namib] is also supported by a PCA undertaken with the EMU (expectation-maximization PCA for Ultra-low Coverage Sequencing Data) approach (44). This approach, which allows for the detection of population structure even with high levels of missing data, shows that KS-Namib can be readily separated from all known major African ancestries, including the southern African–related component identified in ancient (8100 to 2500 B.P.) hunter-gatherers from Malawi (Fig. 6, C and D). A reconstruction of the topology of southern African–related ancestries based on genealogical concordance (3, 45) further shows that the separation of KS-Namib predates the separation of other southern African ancestries, indicating a deep divergence of this component (text S4 and table S8). An early split is further suggested by estimates of divergence time between pairs of populations, showing that the separation of KS-Namib is 13 to 44% older than the split times of the southern African–related ancestries associated with Kx’a (Ju)–, Tuu (Taa)–, and Tuu (!Ui)–speaking populations, assuming an effective population size (Ne) of 20,000 individuals (text S4 and table S9) (3). Together, these results suggest that the Angolan Namib Desert and its surrounding areas preserve the legacy of an extinct, deeply divergent human group with no close matches in extant populations from within and outside southern Africa. |
|
|
|
Post by Admin on Oct 4, 2023 18:41:37 GMT
 Fig. 6. PCA of southern African–related ancestry. (A) Map showing approximate sample locations. The lightest background color shows the desert and xeric shrublands biome. (B to D) PCA built with previously published southern African individuals (see references in table S2) that display <10% of missing data after masking the non–southern African ancestries (B), additionally including Angolan individuals (C), and other relevant modern (see references in table S2) or ancient groups (D) (16, 17). The axis labels include the eigenvalue (in parentheses) for each PC. DISCUSSION The Angolan Namib Desert provides an invaluable framework to examine the history and consequences of contact and admixture between different migratory waves into the wider region of southern Africa. Despite being culturally dominated by Southwest Bantu–speaking cattle herders, the area is remarkable for the presence of several impoverished groups with a peripatetic way of life, who have attracted a considerable degree of ethnographic interest (21, 27, 43, 46, 47). Our results highlight the heterogeneity of the population landscape of the Angolan Namib by showing that, despite their high amounts of Bantu ancestry (~80%), all sampled peripatetic groups display elevated levels of an eastern African ancestry and a previously undocumented southern African–related component (KS-Namib) (Figs. 4 to 6). The co-occurrence of the two pre-Bantu ancestries among all peripatetic groups hints at a complex contact and admixture history. Because the eastern African component has also been detected in Khoe-speaking groups of the Kalahari Basin (Fig. 4), it is likely that it was introduced to southwestern Angola by the ancestors of the present-day Kwepe as part of the Kwadi branch of the Khoe-Kwadi pastoral dispersal. By contrast, KS-Namib has a more restricted distribution, being especially common in the Angolan Namib and appearing in residual amounts in other groups of southwestern Africa, including the Damara from Namibia who are historically linked to a peripatetic way of life (Fig. 5). This distribution suggests that KS-Namib was more likely associated with a resident foraging population of southwest Africa than with migrants from elsewhere. While at present no hunter-gatherers resembling Kx’a- and Tuu-speaking groups exist in the Angolan Namib, an early account by 16th-century traveler Duarte Pacheco-Pereira states that the areas around the Kuroka River mouth were then inhabited by nomadic groups who lived on fishing and built houses from whale ribs that they covered with seaweed (48). This description is reminiscent of coastal foragers often referred to as “Strandlopers” who once lived near the coast in Namibia and South Africa but went extinct during the 19th century (49). Although historical records note them to be Khoekhoe-speaking, their culture differed from the herding groups further inland, and their origins may ultimately trace to resident hunter-gatherer groups, as suggested by the long history of maritime foraging in southern Africa (50). An ancient, prepastoral origin of southern African marine foragers was recently supported by genome-wide data from a 2241- to 1965-ka-old skeleton excavated at St. Helena Bay on the coast of South Africa (16, 51). However, the genetic profile of this individual is close to contemporary Tuu-speaking hunter-gatherers from inland areas of southern Africa and not to the KS-Namib component (Fig. 6). Hence, it is likely that only studies of ancient DNA from the southwestern coast of Angola will be able clarify the ancestral relationships between KS-Namib and extinct forager populations. While the archaeological record for the Angolan Namib is sparse, it is possible that prehistoric human remains associated with this ancestry may be recovered around shell midden deposits and vestiges of coastal settlements that have been reported in areas close to the Kuroka River mouth (52, 53). An ancient occupation of the Namib coast is also supported by oral stories describing an encounter between the Kwadi-speaking Kwepe and resident peoples who had no fire and ate raw fish on the beach (27, 52, 54). Some anthropologists have equated these resident fishermen with the ancestors of the Kwisi and Twa, because of their present socioeconomic marginalization and historically documented association with hunting and gathering, which contrasts with the Kwepe’s higher reliance on small-scale pastoralism (27, 52, 54). However, while our results show that present-day Twa and Kwisi can be genetically separated from the Kwepe (figs. S4 and S6), the three groups are virtually indistinguishable once the effects of genetic drift are attenuated (Fig. 3 and fig. S12). This pattern suggests that all extant peripatetic groups are equally related to different ancestries, thus challenging any attempts to establish continuity between specific modern populations and ancient foragers, beyond ethnographic considerations. The microcosmos of the Angolan Namib can therefore be considered a highly stratified polyethnic system (55) where groups with different genetic and ethnolinguistic backgrounds admixed but maintained sharp divisions based on their socioeconomic status. This contact profile of southwestern Angola is remarkably similar to other areas of southern Africa where Khoe-Kwadi–speaking migrants encountered resident populations with different linguistic and genetic legacies. A defining characteristic of all these areas is an admixture history, which starts with the fusion between an eastern African ancestry and different resident southern African forager components, later followed by various degrees of admixture with Bantu speakers from the western and eastern streams of the Bantu migrations (19). Considering the available genetic, linguistic, and archaeological data (19, 40, 56), we hypothesize that proto–Khoe-Kwadi speakers split in the northwestern Kalahari, contrary to previous proposals that assume a southwestward movement from an intermediate homeland in northeastern Botswana (43, 57). Following their split, the Khoe-Kwadi diverged into different groups migrating into specific contact areas (figs. S1 and S22): Khoekhoe speakers moved south along the Atlantic coast, encountering !Ui-speaking groups; Kalahari Khoe speakers took an eastward route where they encountered Ju speakers in the northern Kalahari, and Taa and ǂHoan speakers in the central Kalahari; and Kwadi speakers migrated toward southwestern Angola, arriving in areas inhabited by a now extinct foraging group associated with the KS-Namib component. More recently, most Khoe-Kwadi–speaking peoples were further affected by East and West Bantu–speaking populations, adding to their diverse genetic makeups. Together, our results show that contact areas associated with the confluence of different migratory waves can harbor the ancestry of vanished groups predating the arrival of food production in Africa. While the full diversity and geographical extension of these early foragers may ultimately be revealed by ancient DNA, detailed studies of highly admixed small-scale communities can still provide unique opportunities to probe the deep genetic structure of the continent. |
|
|
|
Post by Admin on Oct 9, 2023 22:42:32 GMT
Computer simulation of scavenging by hominins and giant hyenas in the late Early Pleistocene Abstract Consumption of animal-sourced food is an important factor in broadening the diet of early hominins, promoting brain and body growth, and increasing behavioural complexity. However, whether early hominins obtained animal food by scavenging or hunting large mammals remains debated. Sabre-toothed felids have been proposed to facilitate the expansion of early Homo out of Africa into Europe 1.4–0.8 Ma by creating a niche for scavengers in Eurasia as the carcasses abandoned by these felids still contained abundant edible resources. In contrast, it has been argued that the niche for a large scavenger was already occupied in Eurasia by the giant hyena, preventing hominins from utilising this resource. This study shows that sabre-toothed felids generated carcasses rich in edible resources and that hominins were capable of competing with giant hyenas for this resource. The simulation experiments showed that maintaining an optimum group size is essential for the success of the hominin scavenging strategy. Early hominins could outcompete giant hyenas only if they could successfully dispute carcasses with them. Thus, in the presence of a strong competitor, passive scavenging is essentially the same as confrontational scavenging. Introduction Hominins arrived to southern Europe at least 1.4 Ma ago1,2,3,4 and were settled there during the Epivillafranchian (approximately 1.2–0.8 Ma)5. However, the roles of changing climate, palaeogeography, faunal assemblages, and other environmental drivers in their dispersion into Europe are under debate6,7,8,9. A key question is how the large European mammalian fauna, especially the composition of the carnivore guild, influenced the accessibility of the early hominins to animal food resources10,11,12,13. Although the dichotomy of hunting vs. scavenging as the main foraging strategies of early Homo is still unresolved14,15,16,17,18,19,20,21,22,23, scavenging has been a common adaptive behaviour in the genus Homo since its origins24,25. Thus, despite the fact that the first hominins in Europe were likely omnivores8, it may be assumed that scavenging was part of their behavioural repertoire (Supplementary Note S1). The scavenging opportunities for a hominin species in a particular ecological scenario can be determined by the complex interaction of several factors, such as the density, size, and quality of carcasses dispersed around the landscape, which can further be determined by the abundance, behavioural, and morphofunctional characteristics of the predators and by the ecological characteristics and abundance of their potential prey8,26. The other main factors to be considered are the presence of competitors and their ecological characteristics and behaviours. It has been suggested that sabre-toothed felids generated many large carcasses because of their inability to entirely consume their kills27,28, facilitating the survival of early Homo during the Epivillafranchian (Supplementary Note S1). This argument is usually applied to species of the genus Megantereon, but may also be applied to Homotherium if solitary behaviour is assumed. Nevertheless, quantitative estimates of the rate of carcass production by these predators and of the amount of nutrients in the abandoned carcasses are currently lacking. In this scenario, an opposite but equally important role, was played by the giant hyena (Pachycrocuta brevirostris)12,29,30, frequently regarded as a “hyperscavenger” and direct competitor of hominins31. Indeed, it has been claimed that the Fuente Nueva-3 site provides evidence of the direct competition between hominins and giant hyenas for an elephant carcass32. Moreover, it has been suggested that the giant hyena was dependent on the partially consumed carcasses produced by sabre-toothed cats, such that the decline of Pachycrocuta in Europe was linked to the extinction of sabre-toothed cats, particularly Megantereon whitei29,30. If hominins practised a flexible strategy of carrion acquisition, several foraging scenarios are possible15,16,18,19,20,33. It has been proposed that groups of hominins were capable of stealing the kills of large predators (confrontational scavenging or kleptoparasitism)17. Moreover, endurance running was suggested to be an advantage for hominins when competing with giant hyenas for carrion, as discussed in4. However, the real advantages provided by endurance running is a controversial topic34. An alternative strategy would be the passive scavenging of partially or completely defleshed carcasses and interference competition for carrion with giant hyena12,30,35. Group size was likely a key factor for both strategies, as a group of hominins was large enough to defend a carcass from any direct competitor. In this study, quantitative estimates of the nutrients contained in the carcasses abandoned by the main Epivillafranchian large predators are provided. Moreover, the competition for carrion between hominins and giant hyenas is approached here through the energetic costs and returns that this interaction represents for both species. Computer-based simulation experiments were performed to simulate the competition between hominins and giant hyenas, evaluate the feasibility of passive scavenging by hominins, and determine the factors that influenced scavenging in the Epivillafranchian ecosystems of the Iberian Peninsula. We aimed to evaluate the effect of ecosystem carrying capacity on the feasibility of passive scavenging and how the size of the hominin group affected the efficiency of this strategy. Although the simulations are an oversimplification of the trophic niche of hominins, this is a necessary methodology for understanding and examining the competition among hominins and hyenas in a tractable and efficient way36. www.nature.com/articles/s41598-023-39776-1 |
|
|
|
Post by Admin on Oct 11, 2023 21:14:01 GMT
Results
The effects of hominin group size and predator density on the competition for carrion between hominins and giant hyenas in two different ecological scenarios were evaluated using six simulation experiments (Table 1). Each experiment was replicated 70 times (see Methods and Supplementary Methods S1), and all the outputs of the 420 replicates (70 runs × six experiments) are shown in Supplementary Dataset 1. For the experiments, it was assumed that giant hyenas were strict solitary scavengers and hominins competed with them for the carcasses produced by large felids (Homotherium latidens, Megantereon whitei, and Panthera gombaszoegensis) in a trophic strategy of passive scavenging.
Carrion production in the Epivillafranchian
Estimated amounts of energy available in the carcasses abandoned by the three large predators were obtained based on the body weight of their preferred prey, the estimated hunting frequency, and the daily intake rate of the predators, as detailed in the “Methods” section. The values included in the experiments are listed in Table 2. These estimates are in general agreement with the values observed in recent ecosystems (Supplementary Methods S2) and confirm the assertion that the two sabre-toothed species generated far more scavengeable resources than recent and fossil pantherines.
The results for the two scenarios were similar. As expected, the final populations of both hyenas and hominins were larger in experiments with higher predator densities (Fig. 1). Indeed, the main difference between the results of both scenarios was that the available resources were insufficient to sustain a hominin population when the predator density was low and only two sabre-toothed cats were present (Fig. 1b). In the scenario of low resources, only giant hyenas survived, although at a low population density (Fig. 1b). In contrast, the presence of a third predator (P. gombaszoegensis) increased the available carrion enough to sustain the populations of both scavengers in most runs, even at a low predator density (Fig. 1a).
|
|
|
|
Post by Admin on Oct 12, 2023 20:18:39 GMT
Figure 1 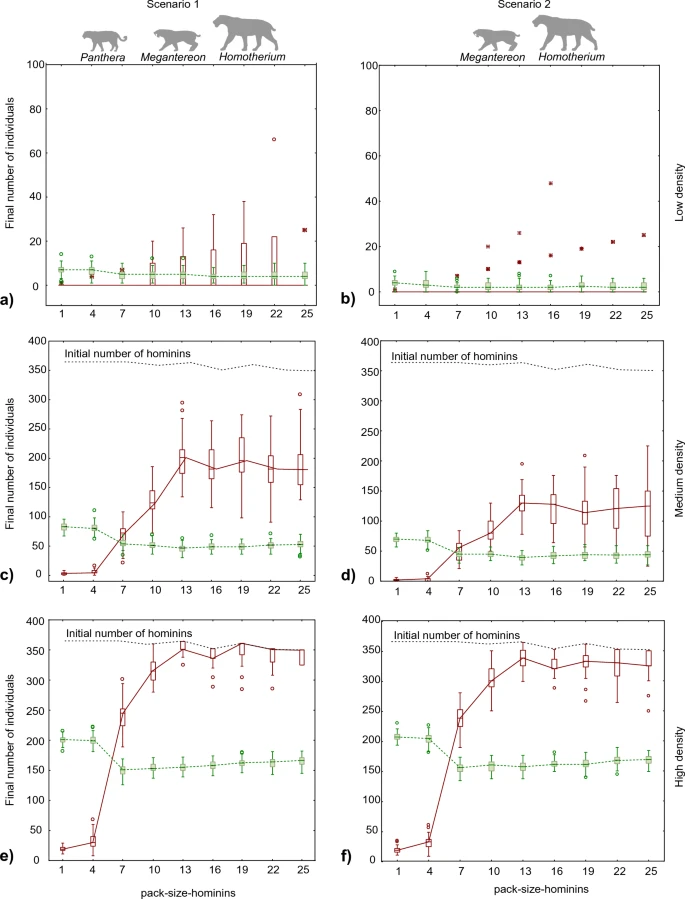 Final number of scavengers in the experiments. Hominins are represented in red (continuous line) and hyenas in green (dashed line). Three experiments were performed by varying the densities of predators for Scenario 1 (a, c, e) and Scenario 2 (b, d, f) from low density (a, b) to medium density (c, d) to high density (e, f). The limits of the boxes correspond to the first and third quartiles; the median is presented with a horizontal line. The whiskers mark the maximum and minimum without outliers or extreme values. Outliers and extreme values are indicated with a white dot and an asterisk, respectively. Competition between hominins and giant hyenas Hominin group size (hominin-pack-size) can predict the competition between hominins and giant hyenas. The final number of hyenas exceeded that of hominins when the size of the hominin group was less than five (Fig. 1), which was arbitrarily set as the threshold necessary for a hominin group to chase away a single hyena. Moreover, hominins could not survive until the end of the simulations when their group size was less than five and the population density of predators was low or medium. When the hominin groups were larger than five, the final population of hominins was larger than that of giant hyenas; however, giant hyenas subsisted under all the conditions tested. The positive effect of increasing hominin group size on the final population of hominins continued until a group size of 13 individuals was reached and levelled off beyond this point (Fig. 1). This pattern can be explained by the fact that a group of more than 10 hominins was necessary to chase away any predator. The differences in the final number of hominins for groups of 13 or more individuals were due to variations in the initial number of groups at the start of the simulation (Fig. 1). This variation was caused by the rounding down of the number of groups to a closer integer after dividing the initial population by the established hominin-pack-size. Energy expenditure is simulated in SCAVCOMP-ABM by letting agents to expend energy at their basal metabolic rate when quiet and at a higher rate when moving (see Methods). Individual hominin energy expenditure decreased with hominin group size, whereas hyena energy expenditure was mostly unaffected by hominin group size (Fig. 2). The effect was difficult to detect at a low predator density in Scenario 2 (Fig. 2c) because the hominins survived to the end of the simulation in very few runs, but it was clearer in Scenario 1 (Fig. 2a). Nevertheless, the pattern appeared to be the same at all three levels of predator density assessed in the experiments. Energy expenditure per hominin decreased steeply with group size until the group size was 13 and increased gently beyond this point. This suggests the existence of an optimal group size that reduces the energy investment required for an activity. The optimum group size was found to be related to the strength necessary to chase away predators and competitors. The decrease in energy expenditure with respect to group size was also influenced by predator density. The higher the predator density, the greater the difference in energy expenditure between individuals in large and small groups of hominins. Similarly, the energy expenditure of hyenas was higher when the predator density was high, since the number of interactions with predators increased (Fig. 2b,d). |
|



