|
|
Post by Admin on Jan 28, 2023 18:11:12 GMT
Analysis of population structure
We performed PCA using the smartpca program from the EIGENSOFT (v.6.01) package90. To avoid bias in the calculation of PCs introduced by high rates of missingness on aDNA, we computed the PCA on 84 modern West Eurasian populations (1,264 individuals genotyped on the Illumina Affymetix Human Origins array) and projected ancient individuals with the option lsqproject.
Admixture analysis with ADMIXTOOLS
We estimated f-statistics using the package ADMIXTOOLS (v.5.1; ref. 91). Depending on their formulation, f-statistics can provide a measure of genetic drift or test for hypotheses of admixture and allele sharing excess. While outgroup f3-test of the form (Mbuti; X, Test)—for X and Test non-African populations—produces high values when X and Test share common drift, f4(Mbuti, Y; X, Test) tests whether X and Y or Test and Y share more alleles than expected from the null hypothesis (X and Test cladal to Y). Therefore, f4-statistics under given settings can provide useful hints for admixture and the possible sources. In addition, computation of f4-statistics comes with a framework for block jackknife estimation of Z-scores, which we use for annotation of significant results (|Z| ≥ 3). We also run admixture f3(A; B, C) that tests whether the allele frequencies of population A are intermediate between B and C, with negative value indicating admixture. Using the information from the f-statistics results we built a framework for running tools qpWave and qpAdm from the same package. A detailed description of the machinery behind these tools is provided in ref. 28. In brief, the method harnesses information about allele frequency differences calculated by multiple f4-statistics that relate a set of reference (right) populations with a set of targets (left) populations. Specifically, qpWave is used to estimate the minimum number of independent gene pools that explain a set of targets from the references. In practice, if two targets are related with the references as one gene pool, then they are cladal (undistinguishable) to the resolution of the references. In qpAdm, which is a derivative of qpWave, this principle is leveraged to model a target population as a mixture of contributions from n source populations. The fit of the full model and the nested simpler models are evaluated and P < 0.05 or 0.01 is generally interpreted as an inadequate explanation of the data. Admixture coefficients outside of the [0,1] range are also evidence of a poor fit of the full model. For the comparison of admixture coefficients from different chromosomes, we computed Z = (coefficientA − coefficientX)/√(s.e.A2 + s.e.X2), where A was any of the 22 autosomes, X the sex chromosome X, s.e. the jackknife standard deviation from the qpAdm and applied a significance threshold of Z ≥ 3.
To further discern differences in ancestries and their admixture coefficients by exploring source populations that potentially serve as proxies of the real sources in terms of time, space as well as the archaeological evidence, we applied a ‘competing’ approach described in previous studies92,93. In this approach, candidate source populations are interchanged between the reference (right) and source (left) populations in the qpAdm setting. If the one placed in the right population is a better proxy for the real source than the one tested in the left ones, the model is expected to fit poorly the data (low P value).
Admixture dating
We used the software DATES (v.753) (https://github.com/priyamoorjani/DATES) to test for exponential decay of local ancestry in a source population given two admixing sources. The decay rate is informative about the time since admixture; thus, the method can effectively date recent admixture events. A detailed explanation of the method is provided47,94,95. We run the method with standard parameters: in Morgan units binsize = 0.001 and fit of decay curve from 0.0045 (lovalfit) to 1 (maxdist) distance bins.
Analysis of biological relatedness
For detection of closely related individuals, we applied the method READ96. In this approach, the coefficient of relatedness [0,1] between two individuals is estimated from their rate of mismatching allele (P0) normalized with the pairwise allele differences among unrelated individuals within the population (α), which is by default calculated as the median from the provided dataset. In this way, the method corrects for SNP ascertainment, marker density, genetic drift and inbreeding. An important implication from this formula is that for given α, the P0 for two identical individuals will be α/2 and hence aDNA data from samples belonging to the same individual can be easily detected. The method also calculates P0 on non-overlapping windows of the genome and computes standard errors.
To detect relatives at a more distant degree, we run lcMLkin97 on the masked versions of bam files with the options -l phred and -g best. This method uses a maximum likelihood framework to infer identical by descent (IBD) on low-coverage DNA sequencing data from genotype likelihoods computed with bcftools. The coefficient of relatedness r is then calculated as k1/2 + k2, with k1 and k2 the probabilities to share one or both alleles IBD, respectively. The method can also distinguish between parent–offspring (k0 = 0) and siblings (k0 ≥ 0, depending on recombination rate) and in theory infer relatedness as distant as fifth degree. However, in low-quality data such as aDNA discrepancies from the expected k0, k1, k2 values are common especially for comparisons relying on <10,000 SNPs31.
To resolve pedigrees that differ in the IBD probabilities (for example, half-siblings or double first cousins), we performed gene imputations with GeneImp (v.1.3; ref. 98) and assessed matching and opposing homozygotes (Supplementary Note 3).
Analysis of ROH
We inferred ROH using hapROH (v.1.0; ref. 50) (https://github.com/hringbauer/hapROH), a method designed to analyse low-coverage aDNA data by leveraging linkage disequilibrium from a panel of modern haplotype references. On 1240K data of at least 0.3× coverage, the method can successfully recover ROH longer than 4 cM. In cases of close parental relatedness, which produce long ROH in the offspring, the method can be efficient for detecting very long ROHs at an even lower coverage. Here, we called ROH in 65 of the Aegean samples (including previously published) with >250,000 SNPs. We simulated individual ROH for a given degree of parental relatedness using the software pedsim (https://github.com/williamslab/ped-sim) as described in Supplementary Section 4, hapROH. We used the embedded functions of the program for plotting the ROH as bars, individual or combined histograms and karyotypes.
Direct AMS radiocarbon dating
Skeletal samples from 38 individuals were submitted to the radiocarbon dating facility of the Klaus-Tschira-Archäometrie-Zentrum at the CEZ Archaeometry gGmbH, Mannheim, Germany, which uses a MICADAS-AMS platform. The same sample from which DNA was extracted was preferred. Collagen was extracted from the bone samples, purified by ultrafiltration (fraction >30 kD) and freeze-dried. Collagen was combusted to CO2 in an elemental analyser and CO2 was converted catalytically to graphite. The 14C ages were normalized to δ13C = −25‰ and were given in BP (before present, meaning years before 1950). The calibration was done using the datasets IntCal13 (ref. 99) and IntCal20 and the software SwissCal 1.0.
|
|
|
|
Post by Admin on Jan 28, 2023 18:14:34 GMT
Supplementary information Supplementary InformationSupplementary Note 1: Details on the archaeological background of the human skeletal material analysed for DNA, with embedded Supplementary Figs. 1–35. Note 2: Modelling and dating of genetic admixture. Note 3: Genotype imputations and pedigree reconstruction for Mygdalia individuals, with embedded Supplementary Figs. 36–38. Reporting SummaryPeer Review FileSupplementary TablesSupplementary Table 1: Library treatment and summary of sequencing statistics for all reported samples before and after their enrichment with the 1240K array capture. Number and percentage of merged reads is reported for paired-end sequenced libraries. Individuals with data combined from different samples are annotated in bold. Supplementary Table 2: Quality control of genome-wide data with information of Y and mitochondrial haplogroups. Low SNP coverage, mitochondrial (mt) contamination estimates from libraries with nu/mt >200, mt haplogroup assignments with low-quality score (0.65–0.8) and X-contamination estimated from <200 polymorphic positions are annotated in italics. Supplementary Table 3: Radiocarbon (14C) dating of 43 samples with calibrated dates presented in 2-sigma range. With few exceptions, the same skeletal element was sampled for aDNA and 14C dating analyses. Samples with <0.5% collagen are typically not analysed as it might alter the 14C ages. The few cases with low collagen are annotated in bold and italics. Supplementary Table 4: Key f4-statistics of the form (Mbuti, Test; Anatolian farmers, Aegean group) that capture excessive allele sharing of Aegean populations with the Test populations (Z ≥ 3; annotated in bold). Published Aegean groups are annotated with an asterisk. Supplementary Table 5: Formal tests of admixture f3(PopA, PopB; PopC). Signals of admixture are detected for LBA Aegean groups with significantly negative f3 values (Z ≥ −3) highlighted in bold. Evidence of admixture of weaker statistical significance (−3 < Z ≤ −2) for the Late Neolithic individuals from Peloponnese is also shown in bold. Published Aegean groups are annotated with an asterisk. Supplementary Table 6. Test of recent admixture for the BA Aegean populations with DATES. For every target, the most robust models (±3 s.e.) and/or fitted decay are highlighted in bold. Supplementary Table 7: Two and three-way qpAdm models for Late Neolithic (LN) to Early/Middle Bronze Age (E/MBA) where sources 2 and 3 are rotated among eight and seven metapopulations from Europe and West Asia, respectively. Models with very poor fit (P ≤ 0.01) and/or infeasible coefficients (±1 s.e.) are annotated in italics, whereas those best fitting in bold. Supplementary Table 8: Two-way qpAdm models for LBA Aegean groups with a local source (source 1) and a battery of Bronze Age European populations (source 2). Models with very poor fit (P ≤ 0.01), infeasible coefficients (±1 s.e.), and low number of SNPs (≤150,000) are annotated in italics. Fitting models are annotated in bold, and those including the corresponding local source or one-way from a non-local source are enclosed in rectangles. Published Aegean groups are annotated with an asterisk. Supplementary Table 9: One- and two-way models for the two LBA groups from Crete (B and C) that require substantial WES-related ancestry. |
|
|
|
Post by Admin on Mar 5, 2023 20:11:37 GMT
 According to a study done by an American researcher at the University of Wales, ancient civilizations may have used celestial navigation methods to travel. Alessandro Berio, a skyscape archaeologist, discovered new evidence that the ancient Minoan civilization developed significant nautical technologies to aid in international sea trade, which is linked to the wealth and expansion of the culture throughout the Mediterranean. Because of its location, Minoan culture was based on open sea navigation and international trade cycles. The Minoan civilization may have relied heavily on celestial star paths above to help them navigate the Mediterranean. According to a study, the Minoan palaces were even placed to face the rising or setting of a few notable stars, serving as their guide to important commercial centers. “It is hypothesized that the orienting of palatial architecture toward star paths and specific sea lanes may have symbolized the special relationships between the palaces and distinct foreign emporia, while also being a source of legitimization of power for the local elite who controlled the ideological and technological frameworks of maritime knowledge,” Berio wrote in the paper. Berio focused his research on the Minoan civilization, a Bronze Age Aegean people who lived on the island of Crete between 2600 – 1100 BC. 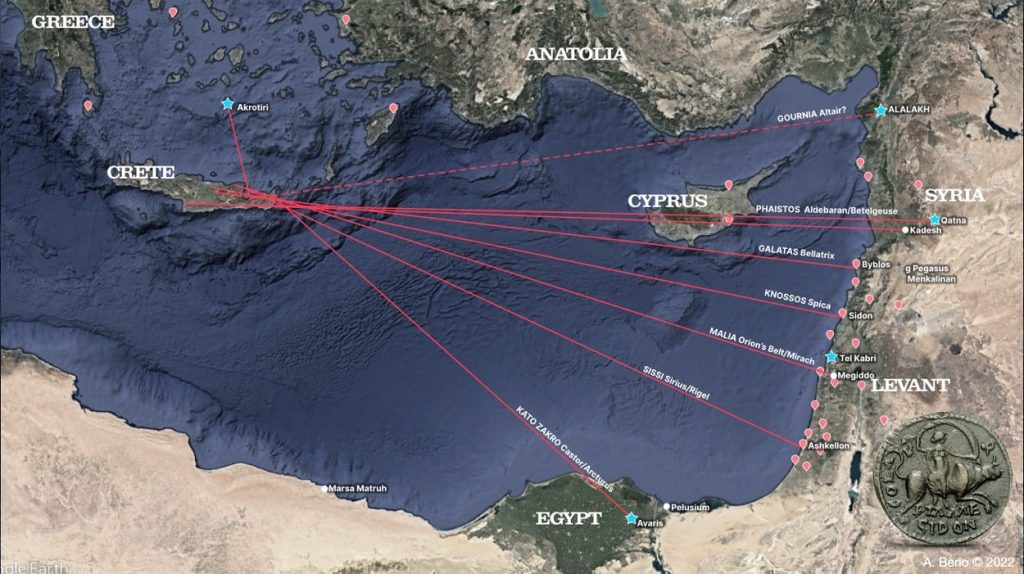 The Pelusiac branch of the River Nile was exactly parallel to the central court of the Minoan trading post. The study found that Knossos, the largest Minoan palace, was perfectly positioned on a “star path” with the constellation of Virgo and the commercial center of Sidon. This alignment may have played a critical role in guiding Minoan sailors to critical trading destinations in Egypt and the Levant. Indeed, the orientation of various palaces toward specific star paths and sea lanes may have symbolized the unique relationship between these palaces and distinct foreign commercial hubs. Furthermore, they may have legitimized the power of the ruling elite, who controlled maritime navigation knowledge and technology, as well as specific sea routes. The research discovered that Minoan sailors may have used star paths or linear constellations to guide them to Mediterranean cities where Minoan artifacts and frescoes bear evidence of trade links between them. The discovery could call into question the previous theory that Homer’s Odyssey was the first historical signal of celestial navigation. We now have more evidence that we need to rewrite history and push back the timeline of human development. The research was published in the Journal Mediterranean Archaeology and Archaeometry. maajournal.com/Issues/2022/Vol22-3/9_Berio_22(3).pdf |
|
|
|
Post by Admin on Jul 30, 2023 20:56:28 GMT
Genetic origins of the Minoans and Mycenaeans Abstract The origins of the Bronze Age Minoan and Mycenaean cultures have puzzled archaeologists for more than a century. We assembled genome-wide data from nineteen ancient individuals, including Minoans from Crete, Mycenaeans from mainland Greece, and their eastern neighbours from southwestern Anatolia. We show that Minoans and Mycenaeans were genetically similar, having at least three quarters of their ancestry from the first Neolithic farmers of western Anatolia and the Aegean1,2, and most of the remainder from ancient populations like those of the Caucasus3 and Iran4,5. However, the Mycenaeans differed from Minoans in deriving additional ancestry from an ultimate source related to the hunter-gatherers of eastern Europe and Siberia6–8, introduced via a proximal source related to either the inhabitants of either the Eurasian steppe1,6,9 or Armenia4,9. Modern Greeks resemble the Mycenaeans, but with some additional dilution of the early Neolithic ancestry. Our results support the idea of continuity but not isolation in the history of populations of the Aegean, before and after the time of its earliest civilizations. Ancient DNA research has traced the principal ancestors of early European farmers to highly similar Neolithic populations of Greece and western Anatolia, beginning in the 7th millennium BCE1,2, but the later history of these regions down to the Bronze Age, a transformational period in the history of Eurasia4,6,9, is less clear. There is limited genetic evidence suggesting migrations from both the east (the area of Iran and the Caucasus), reaching Anatolia by at least ~3,800 BCE4, and the north (eastern Europe and Siberia) contributing ‘Ancient North Eurasian’ ancestry6,10 to all modern Europeans. The timing and impact of these migrations in the Aegean is, however, unknown. During the Bronze Age, two prominent archaeological cultures emerged in the Aegean. The culture of the island of Crete, labelled ‘Minoan’ by Arthur Evans11, was Europe’s first literate civilization, and has been described as ‘Europe’s first major experience of civilization’12. However, the Linear A syllabic ideographic and Cretan hieroglyphic scripts used by this culture remain undeciphered, obscuring its origins. Equally important was the civilization of the ‘Mycenaean’ culture of mainland Greece, whose language, written in the Linear B script, was an early form of Greek13. Cretan influence in mainland Greece and the later Mycenaean occupation of Crete link these two cultures, but the degree of genetic affinity between mainland and Cretan populations is unknown. Greek is related to other Indo-European languages, leading to diverse theories tracing its earliest speakers from the 7th millennium down to ~1,600 BCE, and proposing varying degrees of population change (Supplementary Information, section 1). Genome-wide ancient DNA data provides a new source of information about the people of the Bronze Age, who were first known through the ancient poetic and historical traditions commencing with Homer and Herodotus, later through the disciplines of archaeology and linguistics, and, more recently, by the limited information from ancient mitochondrial DNA14,15. Here we answer several questions. First, do the labels ‘Minoan’ and ‘Mycenaean’ correspond to genetically coherent populations or do they obscure a more complex structure of the peoples who inhabited Crete and mainland Greece at this time? Second, how were the two groups related to each other, to their neighbours across the Aegean in Anatolia, and to other ancient populations from Europe1,2,6,8–10 and the Near East2–5,9,16,17? Third, can inferences about their ancestral origins inform debates about the origins of their cultures? Fourth, how are the Minoans and Mycenaeans related to Modern Greeks, who inhabit the same area today? We generated genome-wide data from 19 ancient individuals (Fig. 1a; Extended Data Table 1; Supplementary Information, section 1). This includes 10 Minoans from Crete, (~2,900–1,700 BCE; labelled Minoan_Odigitria: from Moni Odigitria near the southern coast of central Crete and Minoan_Lasithi: from the cave of Hagios Charalambos in the highland plain of Lasithi in east Crete). From mainland Greece, 4 Mycenaeans were included (~1,700–1,200 BCE; from the western coast of the Peloponnese, from Argolis, and the island of Salamis). An additional individual from Armenoi in western Crete (~1,370–1,340 BCE; labelled Crete_Armenoi) postdates the appearance of Mycenaean culture on the island of Crete. Our dataset also includes a Neolithic sample from Alepotrypa Cave at Diros bay in the southern Peloponnese (~5,400 BCE) adding to previously published samples from northern Greece2 (collectively labelled Greece_N). Finally, it includes 3 Bronze Age individuals (~2,800–1,800 BCE; labelled Anatolia_BA) from Harmanören Göndürle in southwestern Anatolia (Turkey), adding knowledge about genetic variation in Anatolia after the Neolithic/Chalcolithic periods1,2,4,17 (Supplementary Information, section 1). We processed the ancient remains, extracted DNA, and prepared Illumina libraries in dedicated clean rooms (Supplementary Data Table 1; Methods), and, after initial screening for mitochondrial DNA, used in-solution hybridization18 to capture ~1.2 million single nucleotide polymorphisms6,19 on the ancient samples. We assessed contamination by examining the rate at which they matched the mitochondrial consensus sequence (Supplementary Data Table 2) and by the rate at which male samples were heterozygous on the X-chromosome (Methods). We combined the dataset of the 19 ancient individuals with 332 other ancient individuals from the literature, 2,614 present-day humans genotyped on the Human Origins array, and 2 present-day Cretans (Methods). www.ncbi.nlm.nih.gov/pmc/articles/PMC5565772/ |
|
|
|
Post by Admin on Jul 31, 2023 18:56:41 GMT
 Figure 1 Samples and principal components analysis (a) Geographical locations of newly reported ancient data. Lines point to sampling locations; jitter is added to show the number of sampled individual per location. (b) 334 ancient individuals projected onto the first two principal components computed on a sample of 1,029 present-day West Eurasians4,5,10,31, including 30 Modern Greek samples from Greece and Cyprus. We carried out principal components analysis20 (Methods), projecting ancient samples onto the first two principal components inferred from present-day West Eurasian populations10 that form two south-north parallel clines in Europe and the Near East along PC2. Minoans and Mycenaeans are centrally positioned in the PCA (Fig. 1b), framed to the left by ancient populations from mainland Europe and the Eurasian steppe, to the right by ancient populations from the Caucasus and Western Asia, and to the bottom by Early/Middle Neolithic farmers from Europe and Anatolia. The Neolithic samples from Greece cluster with these farmers and are distinct from the Minoans and Mycenaeans. The Bronze Age individuals from southwestern Anatolia are also distinct, intermediate between Anatolian and Levantine populations towards the bottom, and populations from Armenia, Iran, and the Caucasus towards the top. ADMIXTURE analysis (Extended Data Fig. 1) shows that both Minoans and Mycenaeans possess a ‘pink’ genetic component (K=8 and greater) as do Bronze Age southwestern Anatolians, Neolithic Central Anatolians from Tepecik-Çiftlik17, a Chalcolithic northwestern Anatolian1, and western Anatolians from Kumtepe16. This component is maximized in the Mesolithic/Neolithic samples from Iran4,5 and hunter-gatherers from the Caucasus3 (Extended Data Fig. 1). It is not found in the Neolithic of northwestern Anatolia, Greece, or the Early/Middle Neolithic populations of the rest of Europe, only appearing in the populations of the Late Neolithic/Bronze Age in mainland Europe6, introduced there by migration from the Eurasian steppe1,6. Beyond the visual impressions of PCA and ADMIXTURE, we formally tested the relationships between populations from our study and the literature, using f4-statistics of the form f4(X, Y; Test, Chimp) that evaluate whether Test shares more alleles with X or Y. We find that Test populations from Iran, the Caucasus, and eastern Europe share more alleles with Minoans and Mycenaeans than with the Neolithic population of Greece (Extended Data Fig. 2a,b). The Minoans from the Lasithi plateau in the highlands of eastern Crete and from the coast of southern Crete (Extended Data Fig. 2c) are consistent with being a homogeneous population. Mycenaeans differ from these Minoans in sharing significantly fewer alleles with Neolithic people from the Levant, Anatolia, Greece, and mainland Europe (Extended Data Fig. 2d). In comparison, the Bronze Age Anatolians share fewer alleles with ancient Europeans and more with ancient populations of Iran and the Levant (Extended Data Fig. 3). We used f3-statistics of the form f3(Ref1, Ref2; Test) which, if negative, show that Test is admixed from sources related to the Ref1, Ref2 source populations. We do not find significantly negative (Ref1, Ref2) pairs for Minoans or Bronze Age Anatolians (Z>−2.5), but do for Mycenaeans (−4.9<Z<−3.0; Extended Data Fig. 4), involving early farmers from the Levant, Anatolia, Greece, and the rest of Europe as one source, and Iran or the Eurasian steppe or steppe-influenced Europeans as the other. We modelled Bronze Age populations using qpAdm/qpWave6 framework (Methods; Supplementary Information, section 2) which relates a set of ‘left’ populations (admixed population and ancestral source populations) with a set of ‘right’ populations (diverse outgroups) and allows one to test for the number of streams of ancestry from ‘right’ to ‘left’ and to estimate admixture proportions. This analysis shows that all Bronze Age populations from the Aegean and Anatolia derived most (~62–86%) of their ancestry from an Anatolian Neolithic-related population (Table 1). However, they also had a component (~9–32%) of ‘eastern’ (Caucasus/Iran-related) ancestry. It was previously shown that this type of ancestry was introduced into mainland Europe via Bronze Age pastoralists from the Eurasian steppe who were a mix of both eastern European hunter-gathers and populations from the Caucasus and Iran4,6; our results show that it also arrived on its own, at least in the Minoans, without eastern European hunter-gatherer ancestry. This ancestry need not have arrived from regions east of Anatolia, as it was already present during the Neolithic in central Anatolia at Tepecik-Çiftlik17 (Supplementary Information, section 2). The eastern influence in the Bronze Age populations from Greece and southwestern Anatolia is also supported by an analysis of their Y-chromosomes. Four out of five males belonging to Minoans, Mycenaeans, and southwestern Anatolians (Supplementary Information, section 3) belonged to haplogroup J which was rare or non-existent in earlier populations from Greece and western Anatolia which were dominated by Y-chromosome haplogroup G21,2,17. Haplogroup J was present in Caucasus hunter-gatherers3 and a Mesolithic individual from Iran4 and its spread westward may have accompanied the ‘eastern’ genome-wide influence.Table 1 Admixture modelling of Bronze Age populations For each test population, mixture proportions from four source populations with their standard errors are given. Ancestry is inferred from both ‘ultimate’ sources representing the earliest populations, and ‘proximate’ sources representing populations down to the Bronze Age (Supplementary Information, section 2). Column A lists ‘northern’ sources from eastern Europe and Siberia, including the Eurasian steppe; column B lists ‘eastern’ sources from Iran, the Caucasus, and Anatolia (after the Early Neolithic); column C lists ‘local’ sources from Anatolia and the Aegean; column D lists sources from the Levant. For abbreviations of population names see Methods.
Ancestral Sources Mixture Proportions Standard Errors
Test A B C D A B C D A B C D
Ultimate Sources Anatolia_BA CHG Anatolia_N Levant_N 0.319 0.618 0.063 0.029 0.078 0.063
Minoan_Odigitria CHG Anatolia_N 0.144 0.856 0.031 0.031
Minoan_Odigitria Iran_N Anatolia_N 0.137 0.863 0.032 0.032
Minoan_Lasithi MA1 CHG Anatolia_N 0.001 0.152 0.847 0.015 0.021 0.020
Minoan_Lasithi Mota CHG Anatolia_N 0.004 0.154 0.842 0.024 0.026 0.020
Mycenaean AfontovaGora3 CHG Anatolia_N 0.133 0.126 0.741 0.027 0.026 0.024
Mycenaean AfontovaGora3 Iran_N Anatolia_N 0.161 0.086 0.754 0.026 0.025 0.024
Mycenaean EHG Iran_N Anatolia_N 0.065 0.136 0.799 0.016 0.022 0.024
Mycenaean EHG CHG Anatolia_N 0.044 0.176 0.780 0.016 0.023 0.024
Mycenaean MA1 CHG Anatolia_N 0.052 0.159 0.789 0.019 0.026 0.024
Proximate Sources Anatolia_BA Anatolia_ChL Natufian 0.908 0.092 0.039 0.039
Anatolia_BA Anatolia_ChL Levant_BA 0.892 0.108 0.114 0.114
Anatolia_BA Anatolia_ChL Levant_N 0.951 0.049 0.051 0.051
Anatolia_BA Anatolia_ChL Anatolia_N 0.935 0.065 0.062 0.062
Mycenaean Armenia_MLBA Anatolia_N 0.367 0.633 0.020 0.020
Mycenaean Armenia_ChL Anatolia_N 0.441 0.559 0.025 0.025
Anatolia_BA Anatolia_ChL Minoan_Lasithi 0.970 0.030 0.108 0.108
Mycenaean Steppe_MLBA Minoan_Lasithi 0.175 0.825 0.017 0.017
Mycenaean Europe_LNBA Minoan_Lasithi 0.198 0.802 0.019 0.019
Mycenaean Steppe_EMBA Minoan_Lasithi 0.132 0.868 0.014 0.014
The elite Mycenaean individual from the ‘royal’ tomb at Peristeria in the western Peloponnese did not differ genetically from the other three Mycenaean individuals buried in common graves. To identify more proximate sources of the distinctive eastern European/north Eurasian-related ancestry in Mycenaeans, we included later populations as candidate sources (Supplementary Information, section 2), and could model Mycenaeans as a mixture of the Anatolian Neolithic and Chalcolithic-to-Bronze Age populations from Armenia (Table 1). Populations from Armenia possessed some ancestry related to eastern European hunter-gatherers4, so they, or similar unsampled populations of western Asia, could have contributed it to populations of the Aegean. This model makes geographical sense, since a population movement from the vicinity of Armenia could have admixed with Anatolian Neolithic-related farmers on either side of the Aegean. However, Mycenaeans can also be modelled as a mixture of Minoans and Bronze Age steppe populations (Table 1; Supplementary Information, section 2), suggesting that, alternatively, ‘eastern’ ancestry arrived in both Crete and mainland Greece, followed by ~13–18% admixture with a ‘northern’ steppe population in mainland Greece only. Such a scenario is also plausible: first, it provides a genetic correlate for the distribution of shared toponyms in Crete, mainland Greece, and Anatolia discovered by Kretschmer21; second, it postulates a single migration from the east; third, it proposes some gene flow from geographically contiguous areas to the north where steppe ancestry was present since at least the mid-3rd millennium BCE6,9. We validated inferences from qpAdm by treating source populations as ‘ghosts’ and re-estimating mixture proportions4, by examining the correspondence between qpAdm estimates and PCA4 (Extended Data Fig. 5), and by comparing simulated individuals of known ancestry against the Mycenaeans (Extended Data Fig. 6). Geographical structure may have prevented the spread of the ‘northern’ ancestry from the mainland to Crete, contributing to genetic differentiation. Such structure may, in principle, be long-standing, even prior to the advent of the Neolithic in the 7th millennium BCE. Alternatively, both ‘northern’ and ‘eastern’ ancestry may have arrived in the Aegean at any time between the Early Neolithic and the Late Bronze Age. Wider geographical and temporal sampling of pre-Bronze Age populations of the Aegean may better trace the advent of ‘northern’ and ‘eastern’ ancestry in the region. However, sampled Neolithic samples from Greece, down to the Final Neolithic ~4,100 BCE2, do not possess either type of ancestry, suggesting that the admixture we detect probably occurred during the 4th–2nd millennium BCE time window. Other proposed migrations, such as settlement by Egyptian or Phoenician colonists22 are not discernible in our data, as there is no measurable Levantine or African influence in the Minoans and Myceneans, thus rejecting the hypothesis that the cultures of the Aegean were seeded by migrants from the old civilizations of these regions. On the other hand, migrants from areas east or north of the Aegean, while numerically less influential than the locals, may have contributed to the emergence of the 3rd–2nd millennium BCE Bronze Age cultures as ‘creative disruptors’ of local traditions, bearers of innovations, or through cultural interaction with the locals, coinciding with the genetic process of admixture.23 Relative ancestral contributions do not determine the relative roles in the rise of civilization of the different ancestral populations, but, nonetheless, the strong persistence of the Neolithic substratum does suggest a key role for the locals in this process. Phenotype prediction from genetic data has enabled the reconstruction of the appearance of ancient Europeans1,24 who left no visual record of their pigmentation. By contrast, the appearance of the Bronze Age people of the Aegean has been preserved in colourful frescos and pottery, depicting people with mostly dark hair and eyes25. We used the HIrisPlex26 tool (Supplementary Information, section 4) to infer that the appearance of our ancient samples matched the visual representations (Extended Data Table 2), suggesting that art of this period reproduced phenotypes naturalistically. We estimated FST of Bronze Age populations with present-day West Eurasians, finding that Mycenaeans are least differentiated from populations from Greece, Cyprus, Albania, and Italy (Fig. 2), part of a general pattern in which Bronze Age populations broadly resemble present-day inhabitants from the same region (Extended Data Fig. 7). Modern Greeks occupy the intermediate space of the PCA along PC1 (Fig. 1b) between ancient European and Near Eastern populations, like the ones of the Bronze Age. They are not, however, identical to Bronze Age populations, as they are above them along PC2 (Fig. 1b). This is due to the fact that Neolithic farmers share fewer alleles with Modern Greeks than with Mycenaeans (Extended Data Fig. 8), consistent with additional later admixture27,28. |
|



