Post by Admin on Sept 15, 2020 21:11:29 GMT
A forthcoming study from genetic testing giant 23andMe shows that a person’s genetic code could be connected to how likely they are to catch Covid-19 — and how severely they could experience the disease if they catch it. It’s an important confirmation of earlier work on the subject.
People whose blood group is O seemed to test positive for Covid-19 less often than expected when compared to people with any other blood group, according to 23andMe’s data; people who tested positive and had a specific variant of another gene also seemed to be more likely to have serious respiratory symptoms.
The study, which was released on a preprint server and which has not yet been peer-reviewed, could extend and confirm earlier work on the subject; 23andMe’s study relied on a larger dataset than earlier work and included a more diverse set of participants, the company said. Experts who aren’t affiliated with 23andMe praised the study design and the work.
“They clarify further what our data could only vaguely hint at,” said Tom Hemming Karlsen, a physician at Oslo University Hospital who published an article in the New England Journal of Medicine on genetic links with Covid-19 severity in June, and who was not associated with 23andMe’s work.
But the outside experts also cautioned that the research won’t change treatment decisions.
“It doesn’t have practical implications. There’s no treatment decisions that will be made from it — it’s just an interesting finding,” said Jennifer Lighter, a pediatrician and epidemiologist at NYU Langone who was not involved in the research.
Unlike the study Karlsen and his colleagues ran, which only included people with severe Covid-19 symptoms, 23andMe included people who had both mild and severe cases — which allowed them to draw stronger conclusions, Karlsen said.
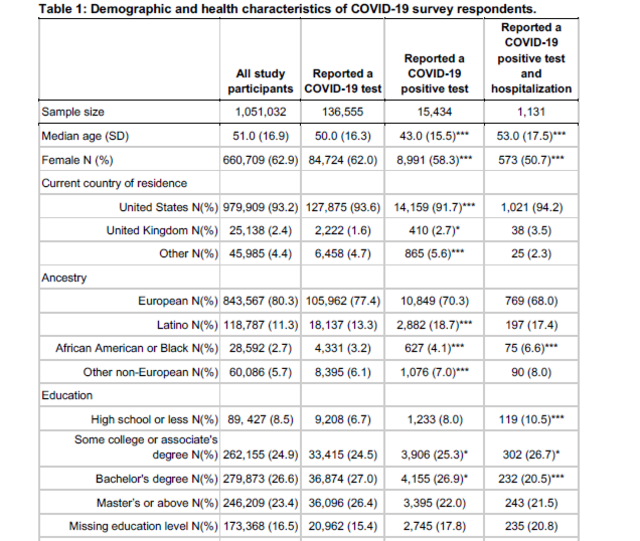
The company’s study participants are also more diverse than Karlsen’s, which only studied people in Spain and Italy. However, the 23andMe study’s demographics still don’t fully reflect the population of the United States. A little more than 11% of the people in 23andMe’s studies said they were Latino; less than 3% said they were Black. (Latinos represent about 16% of the U.S. population, while Black people account for about 13% of the population.)
Both Karlsen and 23andMe’s team found that the genes that code for a person’s blood type seemed to be linked to whether a person would test positive for Covid-19; another section of chromosome 3 — referred to in both papers as chr3p21.31 — seemed to be linked to how severe a person’s response would be to a Covid-19 infection.
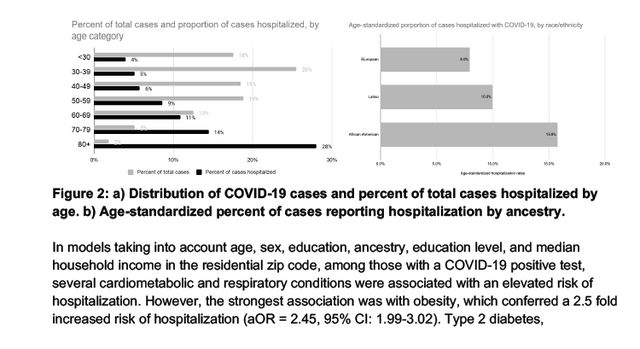
Janie Shelton, a senior scientist at 23andMe and a lead author of the paper, and her colleagues noted that genetic associations did not seem to explain all the differences between populations; public health experts have noticed that people of color seem to be particularly at risk for Covid-19 due to some of the direct and indirect health effects of inequality and discrimination.
Both studies suggested one gene found in that area on chromosome three — SLC6A20 — might be particularly related to worse outcomes; however, it’s not yet clear how a particular gene could make a meaningful difference in a person’s response to an infection.
Both 23andMe and Karlsen ran the same kind of genetic analysis — a genome-wide association study. This particular method, which tries to find similar patterns in the genetics of people with a particular condition, has significant limitations. Scientists have suggested that the method is most useful when used to analyze hundreds of thousands of genomes.
For most scientists, getting that many samples would be difficult and expensive. But 23andMe has an obvious advantage — it has already sequenced more than 12 million people, according to the company’s website; over a million people agreed to participate in the company’s Covid-19 study.
“I do think that because of the power of our large sample size, we were able to detect that association pretty strongly,” said Shelton.
Without a clear understanding of which genes matter — and why — the impact of genetic studies on Covid-19 treatment plans will be limited.
“We’d have to find out why it’s significant — is it significant because it’s affecting blood clotting?” Lighter asked. “Unless we find out why there’s a difference, we wouldn’t target therapies or [adjust] a risk category.”
Trans-ethnic analysis reveals genetic and non-genetic associations with COVID-19 susceptibility and severity
Janie F. Shelton, Anjali J. Shastri, Chelsea Ye, Catherine H. Weldon, Teresa Filshtein-Somnez, Daniella Coker, Antony Symons, Jorge Esparza-Gordillo, The 23andMe COVID-19 Team, Stella Aslibekyan, Adam Auton
doi: doi.org/10.1101/2020.09.04.20188318
Abstract
COVID-19 presents with a wide range of severity, from asymptomatic in some individuals to fatal in others. Based on a study of over one million 23andMe research participants, we report genetic and non-genetic associations with testing positive for COVID-19, respiratory symptoms, and hospitalization. Risk factors for hospitalization include advancing age, male sex, elevated body mass index, lower socio-economic status, non-European ancestry, and pre-existing cardio-metabolic and respiratory conditions. Using trans-ethnic genome-wide association studies, we identify a strong association between blood type and COVID-19 diagnosis, as well as a gene-rich locus on chr3p21.31 that is more strongly associated with outcome severity. While non-European ancestry was found to be a significant risk factor for hospitalization after adjusting for socio-demographics and pre-existing health conditions, we did not find evidence that these two primary genetic associations explain differences between populations in terms of risk for severe COVID-19 outcomes.
People whose blood group is O seemed to test positive for Covid-19 less often than expected when compared to people with any other blood group, according to 23andMe’s data; people who tested positive and had a specific variant of another gene also seemed to be more likely to have serious respiratory symptoms.
The study, which was released on a preprint server and which has not yet been peer-reviewed, could extend and confirm earlier work on the subject; 23andMe’s study relied on a larger dataset than earlier work and included a more diverse set of participants, the company said. Experts who aren’t affiliated with 23andMe praised the study design and the work.
“They clarify further what our data could only vaguely hint at,” said Tom Hemming Karlsen, a physician at Oslo University Hospital who published an article in the New England Journal of Medicine on genetic links with Covid-19 severity in June, and who was not associated with 23andMe’s work.
But the outside experts also cautioned that the research won’t change treatment decisions.
“It doesn’t have practical implications. There’s no treatment decisions that will be made from it — it’s just an interesting finding,” said Jennifer Lighter, a pediatrician and epidemiologist at NYU Langone who was not involved in the research.
Unlike the study Karlsen and his colleagues ran, which only included people with severe Covid-19 symptoms, 23andMe included people who had both mild and severe cases — which allowed them to draw stronger conclusions, Karlsen said.
The company’s study participants are also more diverse than Karlsen’s, which only studied people in Spain and Italy. However, the 23andMe study’s demographics still don’t fully reflect the population of the United States. A little more than 11% of the people in 23andMe’s studies said they were Latino; less than 3% said they were Black. (Latinos represent about 16% of the U.S. population, while Black people account for about 13% of the population.)
Both Karlsen and 23andMe’s team found that the genes that code for a person’s blood type seemed to be linked to whether a person would test positive for Covid-19; another section of chromosome 3 — referred to in both papers as chr3p21.31 — seemed to be linked to how severe a person’s response would be to a Covid-19 infection.
Janie Shelton, a senior scientist at 23andMe and a lead author of the paper, and her colleagues noted that genetic associations did not seem to explain all the differences between populations; public health experts have noticed that people of color seem to be particularly at risk for Covid-19 due to some of the direct and indirect health effects of inequality and discrimination.
Both studies suggested one gene found in that area on chromosome three — SLC6A20 — might be particularly related to worse outcomes; however, it’s not yet clear how a particular gene could make a meaningful difference in a person’s response to an infection.
Both 23andMe and Karlsen ran the same kind of genetic analysis — a genome-wide association study. This particular method, which tries to find similar patterns in the genetics of people with a particular condition, has significant limitations. Scientists have suggested that the method is most useful when used to analyze hundreds of thousands of genomes.
For most scientists, getting that many samples would be difficult and expensive. But 23andMe has an obvious advantage — it has already sequenced more than 12 million people, according to the company’s website; over a million people agreed to participate in the company’s Covid-19 study.
“I do think that because of the power of our large sample size, we were able to detect that association pretty strongly,” said Shelton.
Without a clear understanding of which genes matter — and why — the impact of genetic studies on Covid-19 treatment plans will be limited.
“We’d have to find out why it’s significant — is it significant because it’s affecting blood clotting?” Lighter asked. “Unless we find out why there’s a difference, we wouldn’t target therapies or [adjust] a risk category.”
Trans-ethnic analysis reveals genetic and non-genetic associations with COVID-19 susceptibility and severity
Janie F. Shelton, Anjali J. Shastri, Chelsea Ye, Catherine H. Weldon, Teresa Filshtein-Somnez, Daniella Coker, Antony Symons, Jorge Esparza-Gordillo, The 23andMe COVID-19 Team, Stella Aslibekyan, Adam Auton
doi: doi.org/10.1101/2020.09.04.20188318
Abstract
COVID-19 presents with a wide range of severity, from asymptomatic in some individuals to fatal in others. Based on a study of over one million 23andMe research participants, we report genetic and non-genetic associations with testing positive for COVID-19, respiratory symptoms, and hospitalization. Risk factors for hospitalization include advancing age, male sex, elevated body mass index, lower socio-economic status, non-European ancestry, and pre-existing cardio-metabolic and respiratory conditions. Using trans-ethnic genome-wide association studies, we identify a strong association between blood type and COVID-19 diagnosis, as well as a gene-rich locus on chr3p21.31 that is more strongly associated with outcome severity. While non-European ancestry was found to be a significant risk factor for hospitalization after adjusting for socio-demographics and pre-existing health conditions, we did not find evidence that these two primary genetic associations explain differences between populations in terms of risk for severe COVID-19 outcomes.