|
|
Post by Admin on Apr 14, 2022 20:40:56 GMT
When we added more individuals to create models 2 and 3 (max residuals Z = 3.0 and Z = 3.7), we found that the overall inferred structure and parameters were similar to those of model 1 (Supplementary Tables 9 and 10; see below for specific individuals and regions). The Mota-related and southern-African-related ancestry sources are inferred to split deeply along their respective lineages, meaning that, in some sense, they represent ‘ghost’ populations (without closely related sampled representatives). The central-African-related component is inferred to be closer to Mbuti (including an ancestral admixture event; Supplementary Note 6) than to Aka, and therefore to not split as deeply relative to the initial divergence of the central African forager lineage. Almost all of the additional significant allele-sharing signals that we observed beyond those in model 1 can be attributed to one of the three following causes (Supplementary Table 11): (1) excess relatedness at short-distance scales (see below); (2) admixture from pastoralists and/or farmers more recent than our period of focus (four individuals); or (3) contamination (two individuals). In these cases, we adjusted our final model by (1) allowing shared history (that is, genetic drift) between the relevant individuals; (2) adding the inferred admixture events; or (3) incorporating extra admixture to represent the contamination source (Supplementary Note 6).
For sites in western Kenya, we found that all three individuals in model 3 have excess relatedness beyond the baseline expectation (Fig. 2). The individuals from Jawuoyo (I8808) and Nyarindi (NYA002/NYA003) are the closest, and they can be modelled with Mota-related, central-African-related and southern-African-related ancestry in respective proportions of about 62%, 19% and 19%, while the individual from Kakapel (KPL001) is inferred to have around 12% additional central-African-related ancestry (s.e. of approximately 2–4% with some assumptions) (Fig. 3 and Supplementary Note 6). For north-central Tanzanian sites, again all four individuals have signals of mutual excess allele sharing, with the three individuals from Gishimangeda (I13763, I13982 and I13983) being the closest. One of the three (I13763) shows excess relatedness to non-African individuals, which we interpret as evidence of a small proportion of contamination (Supplementary Notes 5 and 6); otherwise, all four can be fit as a clade with 54%, 12% and 34% Mota-related, central-African-related and southern-African-related ancestry, respectively. Similarly, the three island and coastal individuals (Makangale Cave I1048, Kuumbi Cave I10589, Panga ya Saidi I0595) display excess relatedness, with those from Kuumbi Cave and Panga ya Saidi closest to one another, and with 49% Mota-related, 12% central-African-related and 39% southern-African-related ancestry. These individuals also have ancestry admixed from populations that are associated with food production: Agaw-related for all three, plus western-African-related for Panga ya Saidi (I0595) (Supplementary Note 6).
In contrast to Kenya and Tanzania, we did not observe widespread signals of excess relatedness in Malawi and Zambia. After adjusting for ancestry proportions, most individuals within this geographical cluster are no more related to one another than they are to individuals from Kenya and Tanzania. The only notable exceptions that we found among those in model 3 (Supplementary Note 6) were as follows: (1) among individuals from Fingira (I4426, I4427 and I4468), in particular, two dating to about 6.1 ka; and (2) between the individuals from 9–8 ka from Hora 1 (I2966 and I2967). However, other individuals separated by as little as 100–150 km (Fingira-Hora 1 and Chencherere II-Kalemba) can be fit well with independent mixtures of the same ancestry sources used across the entire study region, including some individuals around 700–1,500 km away. At the same time, the inferred ancestry proportions for the individuals from Malawi and Zambia are quite similar (about 20–30% Mota-related, 5–10% central-African-related and 60–70% southern-African-related), with significant (but small) differences observed for I4426 from Fingira (approximately 11% additional central-African-related ancestry), I4421 from Chencherere (approximately 4% ancestry related to pastoralists), I10726 from Kalemba (approximately 5% less Mota-related ancestry than in Malawi) and I2966 from Hora 1 (a small amount of contamination). We also built an alternative version of our model in which we specified the Malawi individuals as forming a clade descended from a shared three-way admixture event (plus small proportions of additional admixture for the aforementioned individuals) that had only a slightly worsefit—confirming the very similar ancestry proportions among the individuals—but that featured zero shared drift at the base of the clade and almost none on the internal branches (Supplementary Note 6 and Extended Data Fig. 6).
We examined the relationship between geographical distance and genetic relatedness using a new approach based on the residuals of a model assuming that there is no excess shared genetic drift—that is, we observed the similarity of genotypes within pairs of individuals relative to that predicted solely by differential proportions of the three ancestry sources (Methods). Using pairs of individuals from either Kenya and Tanzania, or Malawi and Zambia, together with inter-region pairs to plot the residuals as a function of distance, we found greater relatedness at short distances, but with different length scales for the decay of the fitted curves (about 60 km and about 3 km, respectively) (Extended Data Fig. 7a). Similar patterns are also observed if we omit pairs of individuals that were buried at the same site (Extended Data Fig. 7b). Thus, with the caveats that our sampling is not uniform and that not all of the individuals lived contemporaneously, we found on average that (1) individuals from the same or nearby sites are more closely related than predicted solely on the basis of the broad regional genetic structure, but (2) this relatedness extends only over short distances, particularly within Malawi and Zambia.
For a comparative perspective from contemporaneous ancient foragers in temperate environments, where there are more extensive available data, we performed similar analyses for individuals from Mesolithic Europe (n = 36, about 12–7 ka) (Methods, Supplementary Table 12 and Extended Data Fig. 7c, d). Both western and eastern/northern Europe also show a pattern of greater relatedness at shorter distances; western Europe is similar to Malawi and Zambia in that almost all of the signal comes from same-site pairs, but eastern/northern Europe has a substantially longer geographical decay scale.
Finally, we compared the ancient individuals to the present-day Sandawe and Hadza groups in Tanzania, who historically or recently practiced foraging lifeways. Previous studies have shown that the Hadza and Sandawe have distinctive ancestry from their neighbours, with unusually high proportions of ancestry related to ancient African foragers11,13,14,22. We built an extended version of model 2 including both groups (Extended Data Fig. 8 and Supplementary Note 6). In contrast to the general pattern for ancient individuals, we could not fit Hadza and Sandawe into a simple regional clade, even after accounting for recent admixture that is probably related to incoming pastoralists and farmers (contributing a total of about 41% and about 62% ancestry for these Hadza and Sandawe individuals, respectively). In particular, both were inferred to share a lineage closest to ancient foragers from north-central Tanzania, but the Hadza had excess allele sharing with the Mota individual, while the Sandawe had excess allele sharing with southern African foragers.
|
|
|
|
Post by Admin on Apr 15, 2022 17:37:17 GMT
Effective population sizes
We inferred recent (up to about 500 years before the individual’s birth) ancestral effective population sizes (Ne) for the higher-coverage ancient individuals by scanning for long runs of homozygosity (ROH), which are expected to be present in the genomes of individuals either from populations with small sizes or whose parents have familial relatedness (the latter resulting in especially long ROH) (Methods and Extended Data Fig. 9). The calculation of Ne depends on several factors in addition to census population size; in particular, Ne is a function of both population density and the distance scale of those social interactions that lead to reproduction. All of the ancient individuals are inferred to have at least one long ROH (> 4 centimorgans (cM)), consistent with broad worldwide trends towards smaller population sizes in more ancient societies23. However, the Ne estimates vary by an order of magnitude, from individuals with minimal ROH, suggesting relatively larger population sizes (I5950 (Mota): Ne = 5,470, 95% confidence interval (CI) = 1,237 to unbounded; I8821 (Kisese II): Ne = 2,640, 95% CI = 881–16,424) to those with an ROH of longer than 100 cM, indicative of much smaller population sizes (for example, I8808 (Jawuoyo): Ne = 377, 95% CI = 229–678). Overall, the range is similar to many African forager groups today (Ne, around 500–1,500)24 and towards the low end when compared with present-day population sizes worldwide23.
Discussion
In contrast to previous studies, our results show that a two-way clinal model extending latitudinally from eastern to southern Africa is insufficient to explain observed patterns of genetic variation in ancient sub-Saharan African foragers. Here we demonstrate that central-African-related ancestry (closest to present-day Mbuti among sampled populations), along with Mota-related and southern African-related ancestry, was ubiquitous (in varying proportions) from southwestern Kenya to southeastern Zambia (Fig. 3), with all three components present by at least about 7 ka in Tanzania and about 16 ka in Malawi. Furthermore, when considering ancient African foragers from a wide range of time periods, ecological contexts and archaeological associations, geographical proximity remains the strongest predictor of genetic similarity5,11. Such a pattern may indicate that long-range migrations were rare in the terminal Pleistocene and Holocene, when these individuals lived. This hypothesis is supported by the signals in our admixture graphs of excess genetic relatedness at subregional scales but not at longer-distance scales. Although it is not possible at present to estimate when and how quickly this three-way cline emerged, it must have post-dated both the emergence of the Mota-related lineage around 80–60 ka12,16 and, with respect to the central-African-related ancestry, the split between Aka and Mbuti less than around 50 ka25,26.
Although the observed cline of ancestry remained stable for thousands of years, we propose that it initially arose closer to this split time than to the terminal Pleistocene, and under qualitatively different patterns of mobility and admixture than after it was established. Dispersals, interactions and extensive admixture across eastern and south-central Africa before around 16 ka are evidenced by substantial proportions of ancestry related to the Mota (Ethiopia) individual as far south as Zambia, and ancestry related to southern African foragers as far north as Kenya, in combination with a high degree of homogeneity of ancestry in each subregion after that time. If patterns of mobility and social interactions had remained consistent throughout the Late Pleistocene and Holocene, we would expect to find broad evidence of longer-range ancestry connections within eastern and south-central Africa and beyond, but we observed only two significant plausible instances among our sampled individuals (involving extra central-African-related ancestry in one individual each from Kenya and Malawi).
However, within the three-way population structure, we observed distinct regional trajectories. Individuals from Kenya and Tanzania form three clusters (western Kenya, north-central Tanzania and coastal/island), with individuals in the same cluster showing excess allele sharing even beyond what would be expected from having similar ancestry proportions. This suggests that there is elevated gene flow within each subregion, on a distance scale estimated as approximately 0–100 km. By contrast, the only signals of elevated relatedness detected for individuals from Malawi and Zambia involve those buried at the same site, and can span 1,000–3,600 years (for example, at Fingira). This pattern is best explained by low average human dispersal/interaction distances during much of the Late Pleistocene and Holocene, with the establishment of the broad-scale ancestry cline followed by, on average, more local interactions that differed by region. We observed a similar pattern in ancient foragers from western Europe, whereas those from northern and eastern Europe show longer distance scales of relatedness. This provides genetic evidence that the average distances between where people lived and where their ancestors lived (and therefore the average distances of human movement, especially with respect to reproductive partners) differed among foragers in different regions.
Our genetic findings offer new insights on demographic processes of the Late Pleistocene to Holocene that were previously studied using bioarchaeological, archaeological and linguistic evidence. Beginning approximately 300 ka, archaeological evidence attests to the long-distance movement of materials such as obsidian, presumably facilitated by social networks27. Exchange intensified through the Late Pleistocene to become a hallmark of the LSA, culminating in elaborate transport networks and shared material culture traditions by the Early Holocene1,4,28,29. However, the extent to which people were moving with objects remains an open question. Our genetic results support a scenario in which human mobility and longer-range gene flow occurred with the development and elaboration of long-distance networks approximately 80–20 ka, contributing to the formation of a population structure that persisted over tens of thousands of years during a period when people were living locally.
Genetic evidence also adds weight to arguments for changing Late Pleistocene interaction spheres, with limited gene flow accompanying changes in behaviour and possibly linguistic boundaries. However, at this juncture, we are unable to assess hypothesized population density shifts, based on heightened evidence for symbolic expression at LSA sites and the appearance and disappearance of specific artefact types8,9,30,31,32. Our genetic estimates of recent effective population size are consistent with those of at least some present-day African foragers24, but they are not good comparators due to demographic pressures recently placed on such groups33. Furthermore, small subpopulations with limited gene flow could result in low ancestral effective population sizes even if the region’s total population is high. Preservation of genetic diversity through the existence of many subpopulations over long time scales could also be a contributor to the high levels of genetic diversity observed in most present-day sub-Saharan African groups.
The LSA archaeological record testifies to the appearance of well-defined, temporally and spatially bounded material culture traditions34,35, a phenomenon that is sometimes referred to as regionalization. Faunal data indicate subsistence intensification after around 20 ka36,37, and linguistic data also suggest shifts toward local interactions, reflected in the fact that, today, communities that are presently or historically associated with foraging in central, eastern and southern Africa speak languages of different families (in central Africa, adopted from recent arrivals). At the same time, past regional connectivity and borrowing was such that linguists previously characterized ‘click’ languages as a single family, and the proposed grouping of Khoe–Kwadi–Sandawe strengthens evidence for longer-distance ties between eastern and southern Africa38,39. Our genetic results confirm that trends toward regionalization extended to human population structure, suggesting that decreasing gene flow accompanied changes in behaviour and possibly language.
Conclusions
Demographic transformations in the past approximately 5,000 years have fundamentally altered regional population structures and largely erased what was, by the Late Pleistocene, a well-established three-way cline of eastern-, southern- and central-African-related ancestry that extended across eastern and south-central Africa. Groups who historically forage have frequently been pushed to marginal environments and have experienced transformative demographic changes, making it difficult to learn about deep history from present-day DNA. Today, Africa houses the greatest human genetic diversity, but undersampling of both living and ancient individuals obscures the origins of this diversity40. We show that aDNA from tropical Africa can survive from the Pleistocene and reveal patterns that could not be inferred from populations that lived even a few millennia later, underscoring the breadth of African genetic diversity and the importance of eastern and south-central Africa as long-term reservoirs of human interaction and innovation.
|
|
|
|
Post by Admin on Apr 18, 2022 23:36:13 GMT
In 2004, the scientific world was shaken by the discovery of fossils from a tiny species of hominin on the Indonesian island of Flores. Labeled Homo floresiensis and dating to the late Pleistocene, the species was apparently a contemporary of early modern humans in this part of Southeast Asia. Yet in certain respects the diminutive hominin resembled australopithecines and even chimpanzees. Twenty years previously, when I began ethnographic fieldwork on Flores, I heard tales of humanlike creatures, some still reputedly alive although very rarely seen. In the words of the H. floresiensis discovery team’s leader, the late Mike Morwood, last at the University of Wollongong in Australia, descriptions of these hominoids “fitted floresiensis to a T.” Not least because the newly described fossil species was assumed to be extinct, I began looking for ways this remarkable resemblance might be explained. The result is a book, Between Ape and Human, available in May 2022. Between Ape and Human also considers general questions, including how natural scientists construct knowledge about living things. One issue is the relative value of various sources of information about creatures, including animals undocumented or yet to be documented in the scientific literature, and especially information provided by traditionally non-literate and technologically simple communities such as the Lio—a people who, 40 or 50 years ago, anthropologists would have called primitive. To be sure, the Lio don’t have anything akin to modern evolutionary theory, with speciation driven by mutation and natural selection. But if evolutionism is fundamentally concerned with how different species arose and how differences are maintained, then Lio people and other Flores islanders have for a long time been asking the same questions.  Lio folk zoology and cosmology also include stories of natural beings, specifically humans, transforming permanently into animals of other kinds. And they do this, in part, by moving into new environments and adopting new ways of life, thus suggesting a qualified Lamarckism. As my fieldwork revealed, such posited changes reflect local observations of similarities and differences between a supposed ancestral species and its differentiated descendants. Like the majority of named categories in Lio animal classification, these derivatives coincide with the species or genera of modern systematics. At the same time, Lio distinguish humans from nonhuman animals in much the same way as do modern Westerners, that is, not just on morphological grounds but by attributing complex expressions of culture, language, and technology exclusively to humans. Like other folk zoologists, the Lio put humans first, most notably as the origin of nonhuman animals, a sort of Darwinism in reverse. In contrast, evolutionary theory puts humans (or hominins) last, just as does the biblical story of Genesis. Yet in all instances, the position confers on Homo sapiens a unique status, thereby separating us from the rest of the animal kingdom. For the Lio, the ape-man’s appearance as something incompletely human makes the creature anomalous and hence problematic and disturbing. For academic scientists, H. floresiensis is similarly problematic, but not so much for its resemblance to H. sapiens; rather, it’s because the species appears very late in the geological record, surviving to a time well after the appearance of modern humans. Whether H. floresiensis would have been any harder (or easier) to accept had it been interpreted as a bipedal ape rather than a species of human is difficult to say. Nevertheless, it’s interesting that Morwood, taking an implicitly unilinear view of hominin evolution and arguing for the species’ inclusion in Homo, spoke of the evidence that the diminutive hominin walked the Earth relatively recently as one “good reason” to classify H. floresiensis in our genus. For this can only mean that, in the view of this author, what survives until recent times has to somehow belong with us. As for ape-men, the Lio identify them as animals. In fact, they are one of several animals that Lio people claim descended from humans. But this classification has nothing to do with geological dating or any paleoanthropological evidence. Instead, Lio people, who distinguish natural from supernatural (or spiritual) beings in essentially the same way religious Westerners do, interpret ape-men as non-human animals with reference to observable features that clearly separate them from invisible spirits; from other, more familiar animals; and, of course, from people. Some features of the ape-men might suggest a scientifically undiscovered species or population of modern apes. But Lio statements mostly count against this hypothesis, as does all we know about the biogeography of eastern Indonesia. Our initial instinct, I suspect, is to regard the extant ape-men of Flores as completely imaginary. But, taking seriously what Lio people say, I’ve found no good reason to think so. What they say about the creatures, supplemented by other sorts of evidence, is fully consistent with a surviving hominin species, or one that only went extinct within the last 100 years. Paleontologists and other life scientists would do well to incorporate such Indigenous knowledge into continuing investigations of hominin evolution in Indonesia and elsewhere. For reasons I discuss in the book, no field zoologist is yet looking for living specimens of H. floresiensis or related hominin species. But this does not mean that they cannot be found. Gregory Forth, now retired, was a professor of anthropology at the University of Alberta for more than three decades. Read an excerpt of Between Ape and Human. THIS ARTICLE WAS FEATURED IN APRIL 2022, ISSUE 2 OF THE DIGEST |
|
|
|
Post by Admin on Jun 13, 2022 12:49:36 GMT
The Origins Of Homo Sapiens With Professor Chris Stringer
28,749 views Jun 11, 2022 'The Origins Of Homo Sapiens With Professor Chris Stringer'
From where did humans originate? What did the earliest humans look like? Why did homo sapiens survive while other hominin species went extinct?
In this filmed episode of The Ancients podcast, we're on location at the Natural History Museum in London as Tristan Hughes delves into a huge topic; the origins of modern humans!
Our guest, Professor Chris Stringer, is a leading expert in human evolution. Chris takes us through his research on the origins of our species, from Neanderthals and the strange hobbit-like hominin that lived in Indonesia, fossil evidence from across the planet, and how the 'Out of Africa' theory of early human dispersal has become more complicated in the light of new research.
|
|
|
|
Post by Admin on Oct 18, 2022 6:38:55 GMT
Paleolithic cuisine was anything but lean and green, according to a study on the diets of our Pleistocene ancestors. For a good 2 million years, Homo sapiens and their ancestors ditched the salad and dined heavily on meat, putting them at the top of the food chain. It's not quite the balanced diet of berries, grains, and steak we might picture when we think of 'paleo' food. But according to a study last year by anthropologists from Israel's Tel Aviv University and the University of Minho in Portugal, modern hunter-gatherers have given us the wrong impression of what we once ate. "This comparison is futile, however, because 2 million years ago hunter-gatherer societies could hunt and consume elephants and other large animals – while today's hunter gatherers do not have access to such bounty," researcher Miki Ben‐Dor from Israel's Tel Aviv University explained in 2021. 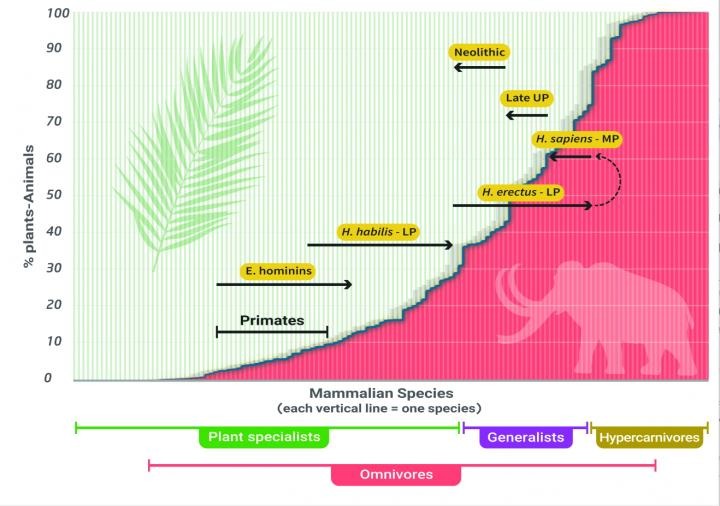 A look through hundreds of previous studies – on everything from modern human anatomy and physiology to measures of the isotopes inside ancient human bones and teeth – suggests we were primarily apex predators until roughly 12,000 years ago. Reconstructing the grocery list of hominids who lived as far back as 2.5 million years ago is made all that much more difficult by the fact plant remains don't preserve as easily as animal bones, teeth, and shells. Other studies have used chemical analysis of bones and tooth enamel to find localized examples of diets heavy in plant material. But extrapolating this to humanity as a whole isn't so straight forward. We can find ample evidence of game hunting in the fossil record, but to determine what we gathered, anthropologists have traditionally turned to modern-day ethnography based on the assumption that little has changed. According to Ben-Dor and his colleagues, this is a huge mistake. "The entire ecosystem has changed, and conditions cannot be compared," said Ben‐Dor. The Pleistocene epoch was a defining time in Earth's history for us humans. By the end of it, we were marching our way into the far corners of the globe, outliving every other hominid on our branch of the family tree. Dominated by the last great ice age, most of what is today Europe and North America was regularly buried under thick glaciers. With so much water locked up as ice, ecosystems around the world were vastly different to what we see today. Large beasts roamed the landscape, including mammoths, mastodons, and giant sloths – in far greater numbers than we see today. Of course it's no secret that Homo sapiens used their ingenuity and uncanny endurance to hunt down these massive meal-tickets. But the frequency with which they preyed on these herbivores hasn't been so easy to figure out. Rather than rely solely on the fossil record, or make tenuous comparisons with pre-agricultural cultures, the researchers turned to the evidence embedded in our own bodies and compared it with our closest cousins. "We decided to use other methods to reconstruct the diet of stone-age humans: to examine the memory preserved in our own bodies, our metabolism, genetics and physical build," said Ben‐Dor. "Human behavior changes rapidly, but evolution is slow. The body remembers." For example, compared with other primates, our bodies need more energy per unit of body mass. Especially when it comes to our energy-hungry brains. Our social time, such as when it comes to raising children, also limits the amount of time we can spend looking for food. We have higher fat reserves, and can make use of them by rapidly turning fats into ketones when the need arises. Unlike other omnivores, where fat cells are few but large, ours are small and numerous, echoing those of a predator. Our digestive systems are also suspiciously like that of animals higher up the food chain. Having unusually strong stomach acid is just the thing we might need for breaking down proteins and killing harmful bacteria you'd expect to find on a week-old mammoth chop. Even our genomes point to a heavier reliance on a meat-rich diet than a sugar-rich one. "For example, geneticists have concluded that areas of the human genome were closed off to enable a fat-rich diet, while in chimpanzees, areas of the genome were opened to enable a sugar-rich diet," said Ben‐Dor. The team's argument is extensive, touching upon evidence in tool use, signs of trace elements and nitrogen isotopes in Paleolithic remains, and dental wear. It all tells a story where our genus' trophic level – Homo's position in the food web – became highly carnivorous for us and our cousins, Homo erectus, roughly 2.5 million years ago, and remained that way until the upper Paleolithic around 11,700 years ago. From there, studies on modern hunter-gatherer communities become a little more useful as a decline in populations of large animals and fragmentation of cultures around the world saw to more plant consumption, culminating in the Neolithic revolution of farming and agriculture. None of this is to say we ought to eat more meat. Our evolutionary past isn't an instruction guide on human health, and as the researchers emphasize, our world isn't what it used to be. But knowing where our ancestors sat in the food web has a big impact on understanding everything from our own health and physiology, to our influence over the environment in times gone by. This research was published in the American Journal of Physical Anthropology. An earlier version of this article was first published in April 2021. |
|


