|
|
Post by Admin on Dec 1, 2020 4:56:05 GMT
Methods This population-based, retrospective cohort study was done across Ontario, Canada, which has universal health care. Existing patient-level data sets for all of Ontario capture all hospitalizations, all emergency department visits, and most laboratory tests for SARS-CoV-2 (Supplement Table 1). These data sets were linked using unique encoded identifiers and analyzed at ICES. All Ontario hospitals submit demographic and clinical information about all inpatient admissions and discharges, including transfers and deaths, using standard diagnostic and procedure codes from the International Classification of Diseases, 10th Revision with Canadian Enhancements. The Canadian Institute for Health Information's Discharge Abstract Database comprises all hospital admissions, and the National Ambulatory Care Reporting System database captures all emergency department visits (Supplement Table 1). The Ontario Health Insurance Plan claims database identifies preexisting health conditions using an International Classification of Diseases, 9th Revision diagnostic code for every outpatient visit. The Ministry of Health's Registered Persons Database contains vital status and demographic information for all persons ever eligible for the Ontario Health Insurance Plan. Residential income quintile and rural residence were identified using Statistics Canada's Census data (Supplement Table 1).  Table 1. Characteristics of 225 386 Persons in Ontario, Canada, With Known ABO Blood Group Who Subsequently Had SARS-CoV-2 Viral RNA Polymerase Chain Reaction Testing Between 15 January and 30 June 2020 Study entry required that a person had ABO blood group assessed between January 2007 and December 2019—before any known international cases of COVID-19—and then subsequently had SARS-CoV-2 viral RNA polymerase chain reaction (PCR) testing between 15 January and 30 June 2020. Persons were excluded if they were not tested for SARS-CoV-2, if sex or birth date were missing, and if they were not Ontario residents. The main study outcome was SARS-CoV-2 infection (a positive result for SARS-CoV-2 on viral RNA PCR testing). The test date was the date on which the SARS-CoV-2 specimen was collected. For any given person, if more than 1 positive specimen date was available, then the earliest specimen with a positive result was considered; otherwise, the earliest specimen with a negative result was considered. A second outcome was a composite of severe COVID-19 illness or death. Severe illness was defined as an admission to an intensive care unit, hospitalization with a length of stay of 7 days or more, or diagnosed myocardial infarction or viral pneumonia, each within 14 days before or after the SARS-CoV-2 test date. Death was captured from 1 day before to up to 14 days after the SARS-CoV-2 test date. We permitted death to precede testing by 1 day to allow for specimen labeling that may occur on the next calendar day. |
|
|
|
Post by Admin on Dec 2, 2020 3:46:22 GMT
Statistical Analysis Baseline characteristics, such as demographics, prior pregnancy, and preexisting health conditions, were assessed relative to the SARS-CoV-2 specimen collection date and presented by ABO blood type. For each study outcome, analyses were done by each ABO blood group (with A as the reference group), O blood group versus all others (reference group), Rh− versus Rh-positive (Rh+) (reference group) status, and O-negative (O−) versus all other ABO and Rh+ blood groups (reference group). Unadjusted probabilities (percentages and 95% CIs) of SARS-CoV-2 infection, as well as severe COVID-19 illness or death, were each estimated in relation to ABO and Rh blood groups. Next, relative risks (RRs) and absolute risk differences (ARDs) based on marginal probabilities of the outcome of interest (also called population-average probabilities of success for exposed and unexposed participants) were calculated using a modified approach to logistic regression analysis developed by Austin (8). The 95% CIs were estimated by bootstrapping with resampling 1000 times (8). The RRs and ARDs were adjusted for age, sex, area-level income quintile, rurality, and local health integration network, each at the time of the SARS-CoV-2 test; for a history of cardiac ischemia or arrhythmia, cancer, or chronic kidney disease diagnosed within 5 years before the SARS-CoV-2 test; and for congestive heart failure or diabetes mellitus diagnosed anytime before the SARS-CoV-2 test (9, 10). For the main outcome of SARS-CoV-2 infection, analyses were further stratified by age younger than 70 years versus 70 years or older (9, 10), and age was excluded from those related multivariable models. In 1 additional analysis, the association between ABO or Rh blood group and risk for severe illness or death was reanalyzed. This analysis was limited to those who tested positive for SARS-CoV-2 and used the modified logistic regression approach described earlier (8). It is known that some persons with SARS-CoV-2 may initially have a false-negative viral RNA PCR test result (11, 12), which may mean that some persons with severe COVID-19 illness could have a negative swab. Accordingly, another additional analysis used multinomial logistic regression to assess the relation between ABO or Rh blood groups and the adjusted odds ratio (aOR) of SARS-CoV-2 negativity with severe illness or death, SARS-CoV-2 positivity without severe illness or death, and SARS-CoV-2 positivity with severe illness or death versus SARS-CoV-2 negativity without severe illness or death (the baseline category). Adjusted models were run with the same covariates as in the aforementioned modified logistic regression models. Persons who have ABO testing may differ from those who do not. Accordingly, we assessed all persons who had SARS-CoV-2 testing in Ontario during the study period and contrasted the characteristics and outcomes among those whose ABO blood group was known versus not known before SARS-CoV-2 testing, expressed as standardized differences. Statistical significance was set at a 2-sided P value of less than 0.05, and analyses were planned a priori. Statistical analyses were done using SAS, version 9.4 for UNIX (SAS Institute). PROC LOGISTIC was used to compute adjusted RRs (aRRs) and ARDs from logistic regression models using a marginal probabilities approach (https://support.sas.com/resources/papers/proceedings11/345-2011.pdf). Role of the Funding Source The funding sources played no role in the design, conduct, or reporting of this study.  Results Among 2 659 328 persons who had an ABO blood group test from January 2007 to December 2019, a total of 2 432 155 did not have a SARS-CoV-2 laboratory test in the subsequent period of observation (Appendix Figure 1). In total, 225 556 persons were included in the final cohort. Of these, 36.3% had blood type A, 4.5% had type AB, 14.9% had type B, and 44.3% had type O (Table 1). The proportion with Rh− status was 13.1%. Mean age was 53.8 years, and about 29% were men. Within 5 years before SARS-CoV-2 specimen collection, about 13% to 15% of persons had preexisting cardiac disease, 11% had chronic kidney disease, 21% had anemia, and 27% to 29% had cancer (Table 1). Asthma (18% to 21%), chronic obstructive pulmonary disease (13% to 17%), and heart failure (10% to 11%) were prevalent, in addition to dementia or frailty (33% to 38%), diabetes mellitus (21%), and chronic hypertension (39% to 41%) at any preceding time. |
|
|
|
Post by Admin on Dec 3, 2020 4:07:04 GMT
SARS-CoV-2 Infection The lowest unadjusted probability of SARS-CoV-2 infection was among the O− blood group (2.1% [95% CI, 1.8% to 2.3%]), and the highest was in the B-positive blood group (4.2% [CI, 4.0% to 4.5%]) (Table 2). Table 2. Unadjusted Probabilities of SARS-CoV-2 Infection or Severe COVID-19 Illness or Death, Each in Relation to ABO and Rh Blood Groups  The aRR for SARS-CoV-2 infection was higher with blood type AB than with type A (1.15 [CI, 1.03 to 1.28]) and B (1.21 [CI, 1.13 to 1.29]) and slightly lower with type O (0.95 [CI, 0.91 to 1.01]) (Table 3). The aRR was 0.88 (CI, 0.84 to 0.92; ARD, −3.9 per 1000 [CI, −5.4 to −2.5]) when comparing O versus all other blood groups. An Rh− status seemed protective against SARS-CoV-2 infection (aRR, 0.79 [CI, 0.73 to 0.85]; ARD, −6.8 per 1000 [CI, −8.9 to −4.7]), especially for those who were O− (aRR, 0.74 [CI, 0.66 to 0.83]; ARD, −8.2 per 1000 [CI, −10.8 to −5.3]). In analyses stratified by age, the relative protective effects of O, Rh−, and O− blood groups were more pronounced among those younger than 70 years than those aged 70 years or older (Appendix Figure 2). Table 3. Association Between ABO, Rh, and ABO-Rh Blood Groups and Risk for SARS-CoV-2 Infection 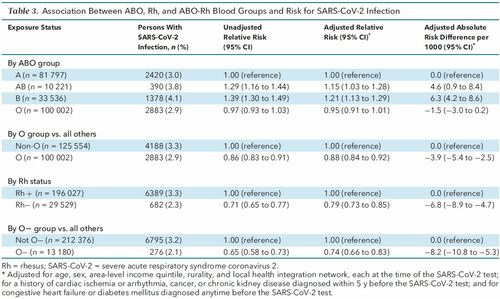 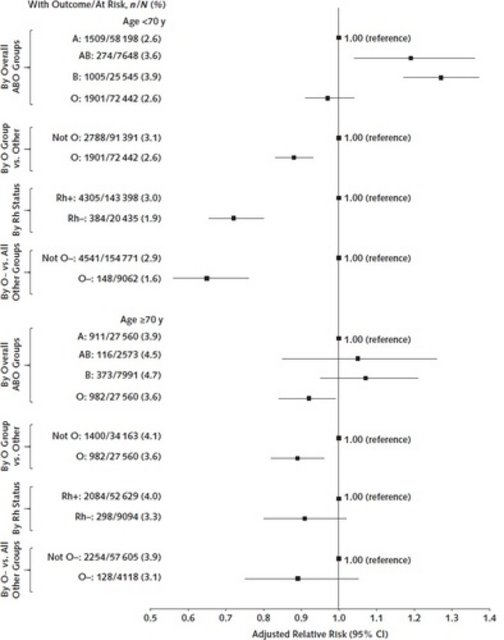 Appendix Figure 2. Risk for SARS-CoV-2 infection associated with ABO, Rh, and ABO-Rh blood groups, stratified by age <70 or ≥70 y. Relative risks are adjusted for sex, area-level income quintile, rurality, and local health integration network, each at the time of SARS-CoV-2 testing, as well as for any history of congestive heart failure, cardiac ischemia or arrhythmia, cancer, diabetes mellitus, or chronic kidney disease diagnosed before the SARS-CoV-2 specimen collection date. Rh = rhesus; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2. |
|
|
|
Post by Admin on Dec 4, 2020 3:47:44 GMT
Severe COVID-19 Illness There were 1328 cases of COVID-19 with severe illness or death, with higher probabilities among AB and B blood groups as well as those who were Rh+ (Table 2). A breakdown of the components of the composite outcome of severe COVID-19 illness or death is shown in Supplement Table 2 (available at Annals.org). Those with blood type B were at significantly higher risk for severe illness than those with type A (aRR, 1.21 [CI, 1.04 to 1.40]; ARD, 1.2 per 1000 [CI, 0.2 to 2.2]) (Table 4). The aRR was 0.87 (CI, 0.78 to 0.97; ARD, −0.8 per 1000 [CI, −1.4 to −0.2]) when comparing O blood group versus all others (Table 4). Compared with Rh+ blood type, Rh− had a lower aRR of severe COVID-19 illness or death (0.82 [CI, 0.68 to 0.96]). Type O− blood did not seem protective against severe COVID-19 illness or death (aRR, 0.84 [CI, 0.64 to 1.07]) (Table 4). Table 4. Association Between ABO, Rh, and ABO-Rh Blood Groups and Risk for Severe COVID-19 Illness or Death 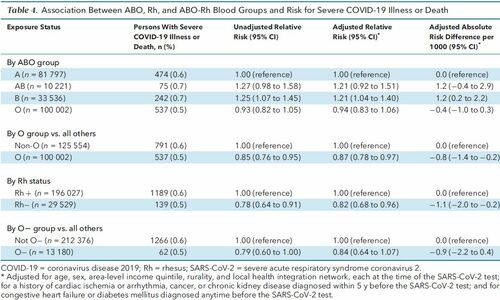 In the first additional analysis, restricted to 7071 persons who tested positive for SARS-CoV-2, there was no observed association between blood group and the risk for severe illness or death (Table 5). However, in a more thorough analysis of all 225 556 patients, including those with a negative SARS-CoV-2 test result, type O blood versus others was protective against SARS-CoV-2 positivity without severe illness or death (aOR, 0.89 [CI, 0.84 to 0.94]) and also SARS-CoV-2 positivity with severe illness or death (aOR, 0.87 [CI, 0.78 to 0.97]) (Table 6). A similar pattern was seen for Rh− status, with respective aORs of 0.80 (CI, 0.73 to 0.87) and 0.82 (CI, 0.68 to 0.98), as well as for O− blood group, with respective aORs of 0.72 (CI, 0.63 to 0.83) and 0.84 (CI, 0.65 to 1.08) (Table 6). Table 5. Association Between ABO, Rh, and ABO-Rh Blood Groups and Risk for Severe COVID-19 Illness or Death, Restricted to 7071 Persons Who Tested Positive for SARS-CoV-2 Infection  Table 6. Association Between ABO, Rh, and ABO-Rh Blood Groups and Risk for Being SARS-CoV-2–Negative With Severe Illness or Death, SARS-CoV-2–Positive Without Severe Illness or Death, or SARS-CoV-2–Positive With Severe Illness or Death, Each in Contrast to Having Neither SARS-CoV-2 Infection nor Severe Illness or Death* Among those who had SARS-CoV-2 testing in the study period, there were some differences in those whose antecedent ABO blood group was known or unknown (Supplement Table 3). For example, those with a known blood type were slightly older, were less likely to be male, and had more comorbidities than those whose ABO status was unknown. However, subsequent rates of SARS-CoV-2 infection or related severe illness did not differ appreciably by ABO status (Supplement Table 3). |
|
|
|
Post by Admin on Dec 4, 2020 21:03:32 GMT
Discussion
In this study, which was done within a universal health care system with widespread SARS-CoV-2 testing, O and Rh− blood groups were associated with a slightly lower risk for SARS-CoV-2 infection as well as severe COVID-19 illness or death.
Our study has several strengths. Testing policies for SARS-CoV-2 have evolved during the Canadian pandemic, from mostly symptomatic persons to broader population screening (https://bit.ly/3e2ar0U); yet, no directive was based on a person's blood group, and the observed subsequent rates of SARS-CoV-2 infection were similar by antecedent ABO test status (Supplement Table 3, lower rows). Persons with early SARS-CoV-2 infection may have a false-negative viral RNA PCR test result (11, 12) and become severely ill days later. This possibility was handled by our second additional analysis considering the outcome of SARS-CoV-2 negativity with severe illness or death, in which there was no important variation in that outcome by blood group (Table 6).
Our study also has limitations. Selection bias was reduced by the requirement that ABO status precede SARS-CoV-2 testing and by further covariate adjustment. Even so, it is possible that those most susceptible to severe COVID-19 illness, such as an elderly resident living in a long-term care facility, died before arriving at the hospital for SARS-CoV-2 testing or died without antecedent symptoms of COVID-19 illness (13). This could be true given that a protective effect from O and Rh− blood type was less pronounced in those older than 70 years (Appendix Figure 2). If O or Rh− blood type is truly protective against SARS-CoV-2 infection, then it is possible that a person who was type O or Rh− would remain asymptomatic and thus not even have viral testing. Accordingly, a study from a setting in which universal screening was done may optimally handle some of these potential issues related to selection bias.
Our study findings align with prior work. In 1 study from China of 2173 patients with COVID-19 and 27 080 unmatched control participants, the unadjusted OR for COVID-19 was 0.67 (CI, 0.60 to 0.75) when comparing O versus non-O blood groups (2). In a recent study of 1610 Italian and Spanish patients with COVID-19 and 2205 unmatched control participants, the age- and sex-adjusted OR for mechanical ventilation was 0.65 (CI, 0.53 to 0.79) when comparing O versus other blood groups (3). A non–peer-reviewed study from NewYork–Presbyterian Hospital comprised 14 112 patients who were tested for SARS-CoV-2 and whose age was about 57 years; 62% were women (4). After adjustment for ethnicity, the risk for SARS-CoV-2 infection was no different between ABO blood types but was lower for Rh− (RR, 0.85 [CI, 0.73 to 0.96]). The risk for intubation did not differ by ABO or Rh status, but the risk for death was lower among Rh− patients (RR, 0.44 [CI, 0.21 to 0.74]) (4).
A retrospective study from Turkey comprised 227 patients who tested positive for SARS-CoV-2 on PCR and another 165 possible cases on the basis of computed tomography scans, who were compared with historical population controls (6). The unadjusted OR of SARS-CoV-2 infection was 0.28 (CI, 0.17 to 0.48) in the presence of Rh− blood type. A cross-sectional study at a single Iranian hospital compared 397 admitted patients with PCR-diagnosed COVID-19 to 500 patients with negative COVID-19 blood samples obtained from outpatient and inpatient services (5). The age- and sex-adjusted ORs were 0.68 (CI, 0.50 to 0.92) when comparing O blood group versus others and 0.91 (CI, 0.58 to 1.43) when comparing Rh− versus Rh+. Moreover, among the 397 hospitalized patients with COVID-19, neither O (aOR, 1.17 [CI, 0.73 to 1.89]) nor Rh− (aOR, 0.70 [CI, 0.33 to 1.49]) blood type was associated with a lower risk for admission to the intensive care unit versus a general ward (5). Taken together, the current body of evidence (some still lacking peer review) suggests that O and Rh− blood types may protect against SARS-CoV-2 infection and, possibly, severe COVID-19 illness.
Our findings may have implications for clinicians and policymakers. If O or Rh− blood type is associated with SARS-CoV-2 infection, then that effect is likely small, which should not undermine the importance of other public health and therapeutic measures aimed at reducing viral transmission or progression to severe COVID-19 illness.
This study was done within a multiethnic Canadian province, but participant ethnicity was not known. Among 3.1 million American blood donors, O− was seen in 8.0% of White non-Hispanic donors, 3.9% of Hispanic donors, 3.6% of Black non-Hispanic donors, and 0.7% of Asian donors (14). Given that Black and Asian persons may be at increased risk for SARS-CoV-2 infection and, possibly, worse clinical outcomes than White persons (15), future large-scale epidemiologic research should consider contrasting the risk for SARS-CoV-2 infection between different subpopulations on an international and regional level. For example, the Basque people of Spain are more likely to be O and Rh− (16) and thus would be expected to be at lower risk for SARS-CoV-2 infection. Other epidemiologic research could expand our current knowledge by measuring not only ABO and Rh status but also the presence of erythrocyte alloimmunization—namely, the formation of antibodies against non–self-antigens on erythrocytes (17).
Although we saw a statistically significant association between blood group and severe disease or death, it was also assumed that we had correctly identified severe illness associated with COVID-19. For example, our composite outcome did not include venous thromboembolism, which is a well-described complication of COVID-19 (18). It is also of interest that the O blood group phenotype and genotype is associated with a decreased risk for venous thromboembolism, possibly because O group members have lower plasma levels of procoagulant factor VIII and von Willebrand factor (19). Thus, the moderating effect of ABO status on venous thromboembolism risk among patients with COVID-19 should be tested.
Studies of the accuracy of serologic tests for anti–SARS-CoV-2 immunoglobulins (20) may assess whether there is variation in antibody titers by ABO and Rh status. Furthermore, among ongoing clinical trials of immunotherapy using convalescent plasma or of SARS-CoV-2 vaccines (21), the interaction between participant blood groups and therapeutic efficacy could be measured.
In conclusion, type O blood may be associated with a lower risk for SARS-CoV-2 infection and severe COVID-19 illness or death. At most, a small proportion of SARS-CoV-2 infection or related illness in the entire population could be prevented by some undetermined property conferred by O blood type and, perhaps, further enhanced by Rh− status. Whether this information can influence COVID-19 prevention or treatment strategies remains to be determined.
This article was published at Annals.org on 24 November 2020
|
|








