|
|
Post by Admin on Oct 13, 2016 21:32:18 GMT
www.biorxiv.org/content/biorxiv/early/2018/05/16/322347.full.pdf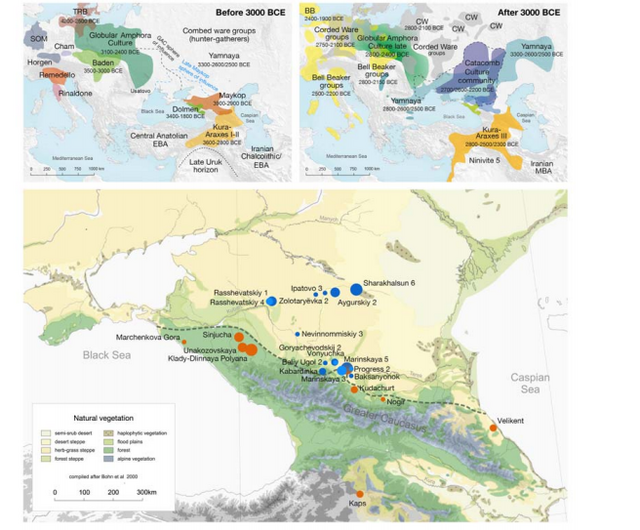 Fig. 1. Map of sites and archaeological cultures mentioned in this study. Admixture into the steppe zone from the south Evidence for interaction between the Caucasus and the Steppe clusters is visible in our genetic data from individuals associated with the later Steppe Maykop phase around 5300-5100 years ago. These ‘outlier’ individuals were buried in the same mounds as those with steppe and in particular Steppe Maykop ancestry profiles but share a higher proportion of Anatolian farmer-related ancestry visible in the ADMIXTURE plot and are also shifted towards the Caucasus cluster in PC space (Fig. 2D). This observation is confirmed by formal D-statistics (Steppe Maykop outlier, Steppe Maykop; X; Mbuti), which are significantly positive when X is a Neolithic or Bronze Age group from the Near East or Anatolia (Supplementary Fig. 4). By modelling Steppe Maykop outliers successfully as a two-way mixture of Steppe Maykop and representatives of the Caucasus cluster (Supplementary Table 3), we can show that these individuals received additional ‘Anatolian and Iranian Neolithic ancestry’, most likely from contemporaneous sources in the south. We estimated admixture time for the observed farmer-related ancestry individuals using the linkage disequilibrium (LD)-based admixture inference implemented in ALDER46, using Steppe Maykop outliers as the test population and Steppe Maykop as well as Kura-Araxes as references. The average admixture time for Steppe Maykop outliers is about 20 generations or 560 years ago, assuming a generation time of 28 years47 (Supplementary Information 6). Contribution of Anatolian farmer-related ancestry to Bronze Age steppe groups In principal component space Eneolithic individuals (Samara Eneolithic) form a cline running from EHG to CHG (Fig. 2D), which is continued by the newly reported Eneolithic steppe individuals. However, the trajectory of this cline changes in the subsequent centuries. Here we observe a cline from Eneolithic_steppe towards the Caucasus cluster. We can qualitatively explain this ‘tilting cline’ by developments south of the Caucasus, where Iranian and Anatolian/Levantine Neolithic ancestries continue to mix, resulting in a blend that is also observed in the Caucasus cluster, from where it could have spread onto the steppe. The first appearance of ‘Near Eastern farmer related ancestry’ in the steppe zone is evident in Steppe Maykop outliers. However, PCA results also suggest that Yamnaya and later groups of the West Eurasian steppe carry some farmer related ancestry as they are slightly shifted towards ‘European Neolithic groups’ in PC2 (Fig. 2D) compared to Eneolithic steppe. This is not the case for the preceding Eneolithic steppe individuals. The tilting cline is also confirmed by admixture f3-statistics, which provide statistically negative values for AG3 as one source and any Anatolian Neolithic related group as a second source peer-reviewed) is the author/funder. Detailed exploration via D-statistics in the form of D(EHG, steppe group; X, Mbuti) and D(Samara_Eneolithic, steppe group; X, Mbuti) show significantly negative D values for most of the steppe groups when X is a member of the Caucasus cluster or one of the Levant/Anatolia farmer-related groups (Supplementary Figs. 5 and 6). In addition, we used f- and D-statistics to explore the shared ancestry with Anatolian Neolithic as well as the reciprocal relationship between Anatolian- and Iranian farmer-related ancestry for all groups of our two main clusters and relevant adjacent regions (Supplementary Fig. 4). Here, we observe an increase in farmer-related ancestry (both Anatolian and Iranian) in our Steppe cluster,ranging from Eneolithic steppe to later groups. In Middle/Late Bronze Age groups especially to the north and east we observe a further increase of Anatolian farmer-related ancestry consistent with previous studies of the Poltavka, Andronovo, Srubnaya and Sintashta groups23, 27 and reflecting a different process not especially related to events in the Caucasus. 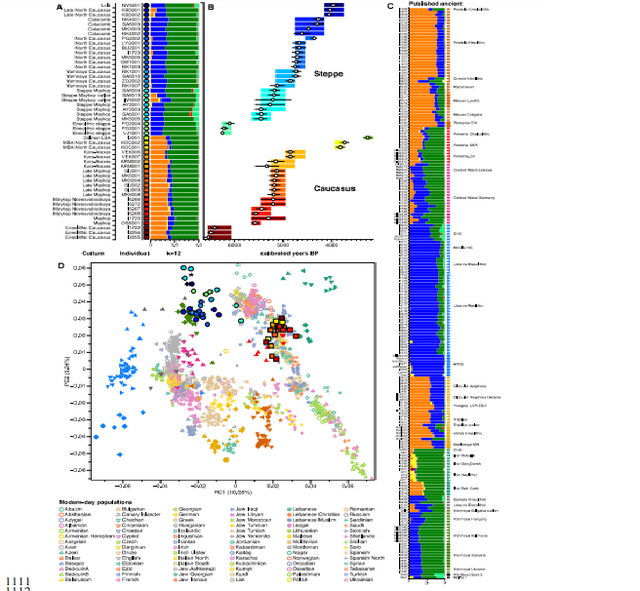 Fig. 2. ADMIXTURE and PCA results, and chronological order of ancient Caucasus individuals. (a) ADMIXTURE results (k=12) of the newly genotyped individuals (filled symbols with black outlines) sorted by genetic clusters (Steppe and Caucasus) and in chronological order (coloured bars indicate the relative archaeological dates, (b) white circles the mean calibrated radiocarbon date and the errors bars the 2-sigma range. (c) ADMIXTURE results of relevant prehistoric individuals mentioned in the text (filled symbols) and (d) shows these projected onto a PCA of 84 modern-day West Eurasian populations (open symbols). The exact geographic and temporal origin of this Anatolian farmer-related ancestry in the North Caucasus and later in the steppe is difficult to discern from our data. Not only do the Steppe groups vary in their respective affinity to each of the two, but also the Caucasus groups, which represent potential sources from a geographic and cultural point of view, are mixtures of them both23. We therefore used qpWave and qpAdm to explore the number of ancestry sources for the Anatolian farmer-related component to evaluate whether geographically proximate groups plausibly contributed to the subtle shift of Eneolithic ancestry in the steppe towards those of the Neolithic groups. Specifically, we tested whether any of the Eurasian steppe ancestry groups can be successfully modelled as a two-way admixture between Eneolithic steppe and a population X derived from Anatolian- or Iranian farmer-related ancestry, respectively. Surprisingly, we found that a minimum of four streams of ancestry is needed to explain all eleven steppe ancestry groups tested, including previously published ones (Fig. 2; Supplementary Table 12). Importantly, our results show a subtle contribution of both Anatolian farmer-related ancestry and WHG-related ancestry (Fig.4; Supplementary Tables 13 and 14), which was likely contributed through Middle and Late Neolithic farming groups from adjacent regions in the West. A direct source of Anatolian farmer-related ancestry can be ruled out (Supplementary Table 15). At present, due to the limits of our resolution, we cannot identify a single best source population. However, geographically proximal and contemporaneous groups such as Globular Amphora and Eneolithic groups from the Black Sea area (Ukraine and Bulgaria), which represent all four distal sources (CHG, EHG, WHG, and Anatolian_Neolithic) are among the best supported candidates (Fig. 4; Supplementary Tables 13,14 and 15). Applying the same method to the subsequent North Caucasian Steppe groups such as Catacomb, North Caucasus, and Late North Caucasus confirms this pattern (Supplementary Table 17). 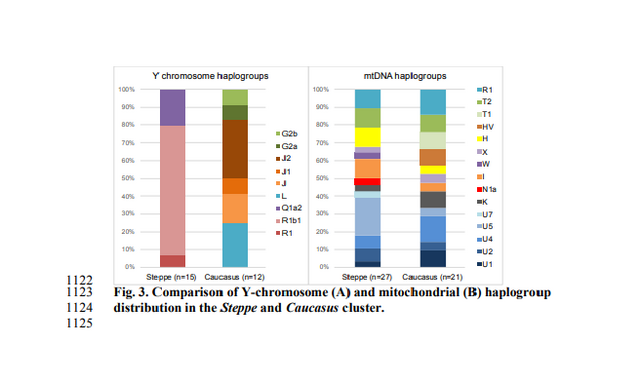 Using qpAdm with Globular Amphora as a proximate surrogate population (assuming that a related group was the source of the Anatolian farmer-related ancestry), we estimated the contribution of Anatolian farmer-related ancestry into Yamnaya and other steppe groups. We find that Yamnaya individuals from the Volga region (Yamnaya Samara) have 13.2±2.7% and Yamnaya individuals in Hungary 17.1±4.1% Anatolian farmer-related ancestry (Fig.4; Supplementary Table 18)– statistically indistinguishable proportions. Replacing Globular Amphora by Iberia Chalcolithic, for instance, does not alter the results profoundly (Supplementary Table 19). This suggests that the source population was a mixture of Anatolian farmer-related ancestry and a minimum of 20% WHG ancestry, a profile that is shared by many Middle/Late Neolithic and Chalcolithic individuals from Europe of the 3rd 448 millennium BCE analysed thus far. To account for potentially un-modelled ancestry from the Caucasus groups, we added ‘Eneolithic Caucasus’ as an additional source to build a three-way model. We found that Yamnaya Caucasus, Yamnaya Ukraine Ozera, North Caucasus and Late North Caucasus had likely received additional ancestry (6% to 40%) from nearby Caucasus groups (Supplementary Table 20). This suggests a more complex and dynamic picture 455 of steppe ancestry groups through time, including the formation of a local variant of steppe ancestry in the North Caucasian steppe from the local Eneolithic, a contribution of Steppe Maykop groups, and population continuity between the early Yamnaya period and the Middle Bronze Age (5300-3200 BP, 3300-2200 calBCE). This was interspersed by additional, albeit subtle gene-flow from the West and occasional equally subtle gene flow from neighbouring groups in the Caucasus and piedmont zones. 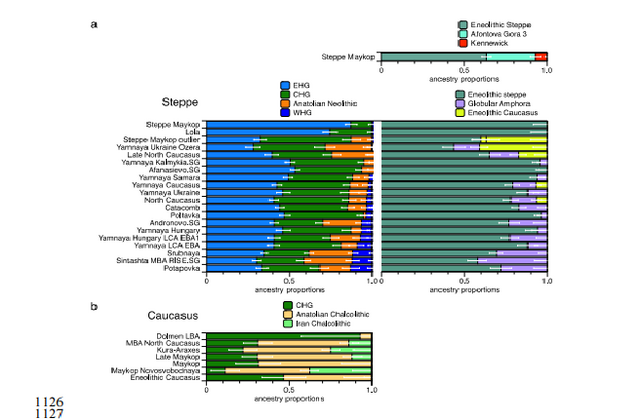 Fig. 4. Modelling results for the Steppe and Caucasus cluster Our results show that at the time of the eponymous grave mound of Maykop, the North Caucasus piedmont region was genetically connected to the south. Even without direct ancient DNA data from northern Mesopotamia, the new genetic evidence suggests an increased assimilation of Chalcolithic individuals from Iran, Anatolia and Armenia and those of the Eneolithic Caucasus during 6000-4000 calBCE23, and thus likely also intensified cultural connections. Within this sphere of interaction, it is possible that cultural influences and continuous subtle gene flow fromthe south formed the basis of Maykop (Fig. 4; Supplementary Table 10). In fact, the Maykop phenomenon was long understood as the terminus of the expansion of South Mesopotamian civilisations in the 4th millennium BCE11, 12, 51. It has been further suggested that along with the cultural and demographic influence the key technological innovations that had revolutionised the late 4th millennium BCE in western Asia had ultimately also spread to Europe52. An earlier connection in the late 5th millennium BCE, however, allows speculations about an alternative archaeological scenario: was the cultural exchange mutual and did e.g. metal rich areas such as the Caucasus contribute substantially to the development and transfer of these innovations53, 54 ? 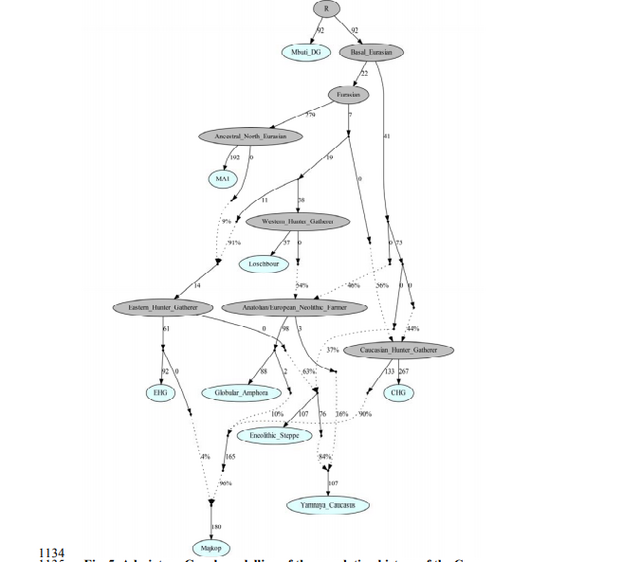 Fig. 5. Admixture Graph modelling of the population history of the Caucasus region. Archaeological arguments would be consonant with both scenarios. Contact between early Yamnaya and late Maykop groups at the end of the 4th 618 millennium BCE is suggested by impulses seen in early Yamnaya complexes. A western sphere of interaction is evident from striking resemblances of imagery inside burial chambers of Central Europe and the Caucasus56 (Supplementary Fig. 9), and particular similarities also exist in geometric decoration patterns in stone cist graves in the Northern Pontic steppe57, on stone stelae in the Caucasus58, and on pottery of the Eastern Globular Amphora Culture, which links the eastern fringe of the Carpathians and the Baltic Sea56. This implies an overlap of symbols with a communication and interaction network that formed during the late 4th 626 millennium BCE and operated across the Black Sea area involving the Caucasus59, 60, and later also involved early Globular Amphora groups in the Carpathians and east/central Europe61. The role of early Yamnaya groups within this network is still unclear57. However, this interaction zone 630 pre-dates any direct influence of Yamnaya groups in Europe or the succeeding formation of the Corded Ware62, 63 and its persistence opens the possibility of subtle bidirectional gene-flow, several centuries before the massive range expansions of pastoralist groups that reached Central Europe in the mid-3rd millennium BCE19. The insight that the Caucasus mountains served not only as a corridor for the spread of CHG/Neolithic Iranian ancestry but also for later gene-flow from the south also has a bearing on the postulated homelands of Proto-Indo-European (PIE) languages and documented gene-flows that could have carried a consecutive spread of both across West Eurasia17, 64 651 . Perceiving the Caucasus as an occasional bridge rather than a strict border during the Eneolithic and Bronze Age opens up the possibility of a homeland of PIE south of the Caucasus, which itself provides a parsimonious explanation for an early branching off of Anatolian languages. Geographically this would also work for Armenian and Greek, for which genetic data also supports an eastern influence from Anatolia or the southern Caucasus. A potential offshoot of the Indo-Iranian branch to the east is possible, but the latest ancient DNA results from South Asia also lend weight to an LMBA spread via the steppe belt21. The spread of some or all of the proto-Indo-European branches would have been possible via the North Caucasus and Pontic region and from there, along with pastoralist expansions, to the heart of Europe. This scenario finds support from the well attested and now widely documented ‘steppe ancestry’ in European populations, the postulate of increasingly patrilinear societies in the wake of these expansions (exemplified by R1a/R1b), as attested in the latest study on the Bell Beaker phenomenon35. bioRxiv preprint first posted online May. 16, 2018; doi: dx.doi.org/10.1101/322347.
|
|
|
|
Post by Admin on Apr 28, 2021 19:49:03 GMT
Populations of hunter-gatherers weathered Ice Age in apparent isolation in Caucasus mountain region for millennia, later mixing with other ancestral populations, from which emerged the Yamnaya culture that would bring this Caucasus hunter-gatherer lineage to Western Europe.  The first sequencing of ancient genomes extracted from human remains that date back to the Late Upper Palaeolithic period over 13,000 years ago has revealed a previously unknown “fourth strand” of ancient European ancestry. This new lineage stems from populations of hunter-gatherers that split from western hunter-gatherers shortly after the ‘out of Africa’ expansion some 45,000 years ago and went on to settle in the Caucasus region, where southern Russia meets Georgia today. Here these hunter-gatherers largely remained for millennia, becoming increasingly isolated as the Ice Age culminated in the last ‘Glacial Maximum’ some 25,000 years ago, which they weathered in the relative shelter of the Caucasus mountains until eventual thawing allowed movement and brought them into contact with other populations, likely from further east. This led to a genetic mixture that resulted in the Yamnaya culture: horse-borne Steppe herders that swept into Western Europe around 5,000 years ago, arguably heralding the start of the Bronze Age and bringing with them metallurgy and animal herding skills, along with the Caucasus hunter-gatherer strand of ancestral DNA – now present in almost all populations from the European continent. The research was conducted by an international team led by scientists from Cambridge University, Trinity College Dublin and University College Dublin. The findings are published today in the journal Nature Communications. “The question of where the Yamnaya come from has been something of a mystery up to now,” said one of the lead senior authors Dr Andrea Manica, from Cambridge’s Department of Zoology. “We can now answer that as we’ve found that their genetic make-up is a mix of Eastern European hunter-gatherers and a population from this pocket of Caucasus hunter-gatherers who weathered much of the last Ice Age in apparent isolation. This Caucasus pocket is the fourth major strand of ancient European ancestry, one that we were unaware of until now,” he said Professor Daniel Bradley, leader of the Trinity team, said: “This is a major new piece in the human ancestry jigsaw, the influence of which is now present within almost all populations from the European continent and many beyond.” Previously, ancient Eurasian genomes had revealed three ancestral populations that contributed to contemporary Europeans in varying degrees, says Manica.  Following the ‘out of Africa’ expansion, some hunter-gatherer populations migrated north-west, eventually colonising much of Europe from Spain to Hungary, while other populations settled around the eastern Mediterranean and Levant, where they would develop agriculture around 10,000 years ago. These early farmers then expanded into and colonised Europe. Lastly, at the start of the Bronze Age around 5,000 years ago, there was a wave of migration from central Eurasia into Western Europe – the Yamnaya. However, the sequencing of ancient DNA recovered from two separate burials in Western Georgia – one over 13,000 years old, the other almost 10,000 years old – has enabled scientists to reveal that the Yamnaya owed half their ancestry to previously unknown and genetically distinct hunter-gatherer sources: the fourth strand. By reading the DNA, the researchers were able to show that the lineage of this fourth Caucasus hunter-gatherer strand diverged from the western hunter-gatherers just after the expansion of anatomically modern humans into Europe from Africa. The Caucasus hunter-gatherer genome showed a continued mixture with the ancestors of the early farmers in the Levant area, which Manica says makes sense given the relative proximity. This ends, however, around 25,000 years ago – just before the time of the last glacial maximum, or peak Ice Age. At this point, Caucasus hunter-gatherer populations shrink as the genes homogenise, a sign of breeding between those with increasingly similar DNA. This doesn’t change for thousands of years as these populations remain in apparent isolation in the shelter of the mountains – possibly cut off from other major ancestral populations for as long as 15,000 years – until migrations began again as the Glacial Maximum recedes, and the Yamnaya culture ultimately emerges. “We knew that the Yamnaya had this big genetic component that we couldn’t place, and we can now see it was this ancient lineage hiding in the Caucasus during the last Ice Age,” said Manica. While the Caucasus hunter-gatherer ancestry would eventually be carried west by the Yamnaya, the researchers found it also had a significant influence further east. A similar population must have migrated into South Asia at some point, says Eppie Jones, a PhD student from Trinity College who is the first author of the paper. “India is a complete mix of Asian and European genetic components. The Caucasus hunter-gatherer ancestry is the best match we’ve found for the European genetic component found right across modern Indian populations,” Jones said. Researchers say this strand of ancestry may have flowed into the region with the bringers of Indo-Aryan languages. The widespread nature of the Caucasus hunter-gatherer ancestry following its long isolation makes sense geographically, says Professor Ron Pinhasi, a lead senior author from University College Dublin. “The Caucasus region sits almost at a crossroads of the Eurasian landmass, with arguably the most sensible migration routes both west and east in the vicinity.” He added: “The sequencing of genomes from this key region will have a major impact on the fields of palaeogeneomics and human evolution in Eurasia, as it bridges a major geographic gap in our knowledge.” David Lordkipanidze, Director of the Georgian National Museum and co-author of the paper, said: “This is the first sequence from Georgia – I am sure soon we will get more palaeogenetic information from our rich collections of fossils.” Reference: E.R. Jones et. al. ‘Upper Palaeolithic genomes reveal deep roots of modern Eurasians.’ Nature Communications (2015). DOI: 10.1038/ncomms9912 |
|
|
|
Post by Admin on Apr 29, 2021 2:44:34 GMT
Upper Palaeolithic genomes reveal deep roots of modern Eurasians
Nature Communications volume 6, Article number: 8912 (2015)
Abstract
We extend the scope of European palaeogenomics by sequencing the genomes of Late Upper Palaeolithic (13,300 years old, 1.4-fold coverage) and Mesolithic (9,700 years old, 15.4-fold) males from western Georgia in the Caucasus and a Late Upper Palaeolithic (13,700 years old, 9.5-fold) male from Switzerland. While we detect Late Palaeolithic–Mesolithic genomic continuity in both regions, we find that Caucasus hunter-gatherers (CHG) belong to a distinct ancient clade that split from western hunter-gatherers ∼45 kya, shortly after the expansion of anatomically modern humans into Europe and from the ancestors of Neolithic farmers ∼25 kya, around the Last Glacial Maximum. CHG genomes significantly contributed to the Yamnaya steppe herders who migrated into Europe ∼3,000 BC, supporting a formative Caucasus influence on this important Early Bronze age culture. CHG left their imprint on modern populations from the Caucasus and also central and south Asia possibly marking the arrival of Indo-Aryan languages.
Introduction
Ancient genomes from Eurasia have revealed three ancestral populations that contributed to contemporary Europeans in varying degrees1. Mesolithic individuals, sampled from Spain all the way to Hungary1,2,3, belong to a relatively homogenous group, termed western hunter-gatherers (WHG). The expansion of early farmers (EF) out of the Levant during the Neolithic transition led to major changes in the European gene pool, with almost complete replacement in the south and increased mixing with local WHG further north1,2,3,4,5. Finally, a later wave originating with the Early Bronze Age Yamnaya from the Pontic steppe, carrying partial ancestry from ancient North Eurasians (ANE) and ancestry from a second, undetermined source, arrived from the east, profoundly changing populations and leaving a cline of admixture in Eastern and Central Europe1,3,6. This view, which was initially based on a handful of genomes, was recently confirmed by extensive surveys of Eurasian samples from the Holocene5,7.
Here, we extend our view of the genetic makeup of early Europeans by both looking further back in time and sampling from the crossroads between the European and Asian continents. We sequenced a Late Upper Palaeolithic (‘Satsurblia’ from Satsurblia cave, 1.4-fold coverage) and a Mesolithic genome (‘Kotias’ from Kotias Klde cave, 15.4-fold) from Western Georgia, at the very eastern boundary of Europe. We term these two individuals Caucasus hunter-gatherers (CHG). To extend our overview of WHG to a time depth similar to the one available for our samples from the Caucasus, we also sequenced a western European Late Upper Palaeolithic genome, ‘Bichon’ (9.5-fold) from Grotte du Bichon, Switzerland. These new genomes, together with already published data, provide us with a much-improved geographic and temporal coverage of genetic diversity across Europe after the Last Glacial Maximum (LGM)8. We show that CHG belong to a new, distinct ancient clade that split from WHG ∼45 kya and from Neolithic farmer ancestors ∼25 kya. This clade represents the previously undetermined source of ancestry to the Yamnaya, and contributed directly to modern populations from the Caucasus all the way to Central Asia.
|
|
|
|
Post by Admin on Apr 29, 2021 5:48:54 GMT
Results Samples, sequencing and authenticity Recent excavations of Satsurblia cave in Western Georgia yielded a human right temporal bone, dated to the Late Upper Palaeolithic 13,132–13,380 cal. BP. Following the approach of Gamba et al.3, extractions from the dense part of the petrous bone yielded sequencing libraries comprising 13.8% alignable human sequence which were used to generate 1.4-fold genome coverage. A molar tooth sampled from a later Mesolithic (9,529–9,895 cal. BP) burial in Kotias Klde, a rockshelter also in Western Georgia showed excellent preservation, with endogenous human DNA content of 76.9%. This was sequenced to 15.4-fold genome coverage. Grotte du Bichon is a cave situated in the Swiss Jura Mountains where a skeleton of a young male of Cro-magnon type was found and dated to the late Upper Palaeolithic 13,560–13,770 cal. BP (for further details on the archaeological context see Supplementary Note 1). A petrous bone sample extraction from this also gave excellent endogenous content at 71.5% and was sequenced to 9.5-fold coverage. The sequence data from each genome showed sequence length and nucleotide misincorporation patterns which were indicative of post-mortem damage and contamination estimates, based on X chromosome and mitochondrial DNA tests (Supplementary Note 2), were <1%, comparable to those found in other ancient genomes2,3,8. Continuity across the Palaeolithic–Mesolithic boundary Kotias and Satsurblia, the two CHG, are genetically different from all other early Holocene (that is, Mesolithic and Neolithic) ancient genomes1,2,3,4,5,6,8,9,10, while Bichon is similar to other younger WHG. The distinctness of CHG can be clearly seen on a principal component analysis (PCA) plot11 loaded on contemporary Eurasian populations1, where they fall between modern Caucasian and South Central Asian populations in a region of the graph separated from both other hunter gatherer and EF samples (Fig. 1a). Clustering using ADMIXTURE software12 confirms this view, with CHG forming their own homogenous cluster (Fig. 1b). The close genetic proximity between Satsurblia and Kotias is also formally supported by D-statistics13, indicating the two CHG genomes form a clade to the exclusion of other pre-Bronze Age ancient genomes (Supplementary Table 2; Supplementary Note 3), suggesting continuity across the Late Upper Palaeolithic and Mesolithic periods. This result is mirrored in western Europe as Bichon is close to other WHG in PCA space (Fig. 1a) and outgroup f3 analysis (Supplementary Fig. 1), belongs to the same cluster as other WHG in ADMIXTURE analysis (Fig. 1b), and forms a clade with other WHG to the exclusion of other ancient genomes based on D-statistics (Supplementary Table 3; Supplementary Note 3). Thus, these new data indicate genomic persistence between the Late Upper Palaeolithic and Mesolithic both within western Europe and, separately, within the Caucasus. Figure 1: Genetic structure of ancient Europe. 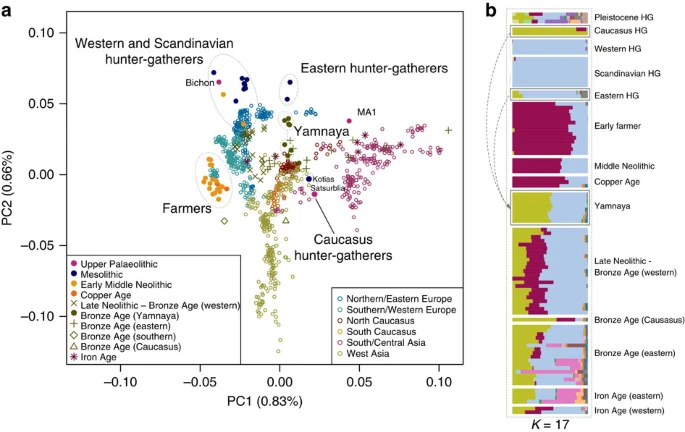 (a). Principal component analysis. Ancient data from Bichon, Kotias and Satsurblia genomes were projected11 onto the first two principal components defined by selected Eurasians from the Human Origins data set1. The percentage of variance explained by each component accompanies the titles of the axes. For context we included data from published Eurasian ancient genomes sampled from the Late Pleistocene and Holocene where at least 200 000 SNPs were called1,2,3,4,5,6,7,9 (Supplementary Table 1). Among ancients, the early farmer and western hunter-gatherer (including Bichon) clusters are clearly identifiable, and the influence of ancient north Eurasians is discernible in the separation of eastern hunter-gatherers and the Upper Palaeolithic Siberian sample MA1. The two Caucasus hunter-gatherers occupy a distinct region of the plot suggesting a Eurasian lineage distinct from previously described ancestral components. The Yamnaya are located in an intermediate position between CHG and EHG. (b). ADMIXTURE ancestry components12 for ancient genomes (K=17) showing a CHG component (Kotias, Satsurblia) which also segregates in in the Yamnaya and later European populations. |
|
|
|
Post by Admin on Apr 30, 2021 1:05:33 GMT
Deep coalescence of early Holocene lineages The geographical proximity of the Southern Caucasus to the Levant begs the question of whether CHG might be related to early Neolithic farmers with Near Eastern heritage. To address this question formally we reconstructed the relationship among WHG, CHG and EF using available high-quality ancient genomes1,3. We used outgroup f3-statistics14 to compare the three possible topologies, with the correct relationship being characterized by the largest amount of shared drift between the two groups that form a clade with respect to the outgroup (Fig. 2a; Supplementary Note 4). A scenario in which the population ancestral to both CHG and EF split from WHG receives the highest support, implying that CHG and EF form a clade with respect to WHG. We can reject a scenario in which CHG and WHG form a distinct clade with respect to EF. The known admixture of WHG with EF1,3,4,5 implies that some shared drift is found between WHG and EF with respect to CHG, but this is much smaller than the shared drift between CHG and EF. Thus, WHG split first, with CHG and EF separating only at a later stage. Figure 2: The relationship between Caucasus hunter-gatherers (CHG), western hunter-gatherers and early farmers. 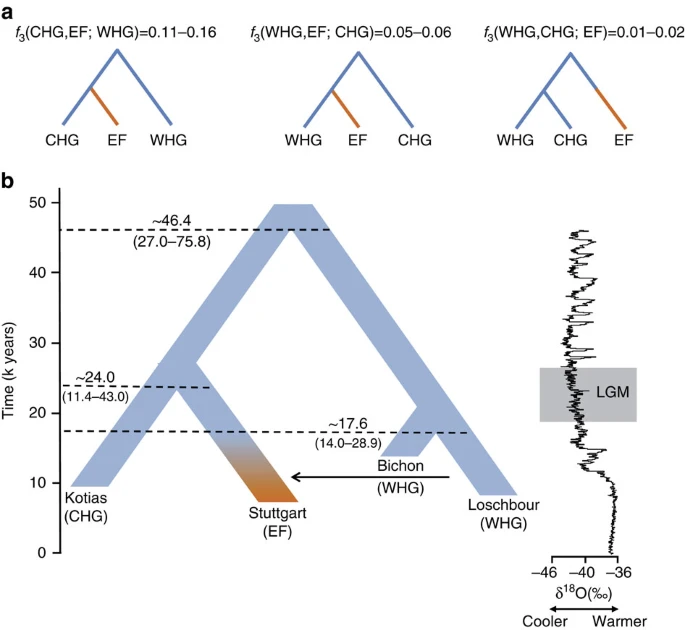 (a). Alternative phylogenies relating western hunter-gatherers (WHG), CHG and early farmers (EF, highlighted in orange), with the appropriate outgroup f3-statistics. (b). The best supported relationship among CHG (Kotias), WHG (Bichon, Loschbour), and EF (Stuttgart), with split times estimates using G-Phocs15. Oxygen 18 values (per mile) from the NGRIP core provide the climatic context; the grey box shows the extent of the Last Glacial Maximum (LGM). We next dated the splits among WHG, CHG and EF using a coalescent model implemented with G-PhoCS15 based on the high-coverage genomes in our data set (Fig. 2b for a model using the German farmer Stuttgart1 to represent EF; and Supplementary Table 5 for models using the Hungarian farmer NE1 (ref. 3)) and taking advantage of the mutation rate recently derived from Ust’-Ishim10. G-Phocs dates the split between WHG and the population ancestral to CHG and EF at ∼40–50 kya (range of best estimates depending on which genomes are used; see Supplementary Table 5 and Supplementary Note 5 for details), implying that they diverged early on during the colonisation of Europe16, and well before the LGM. On the WHG branch, the split between Bichon and Loschbour1 is dated to ∼16–18 kya (just older than the age of Bichon), implying continuity in western Europe, which supports the conclusions from our previous analyses. The split between CHG and EF is dated at ∼20–30 kya emerging from a common basal Eurasian lineage1 (Supplementary Fig. 2) and suggesting a possible link with the LGM, although the broad confidence intervals require some caution with this interpretation. In any case, the sharp genomic distinctions between these post-LGM populations contrasts with the comparative lack of differentiation between the earlier Eurasian genomes, for example, as visualized in the ADMIXTURE analysis (Fig. 1a), and it seems likely that this structure emerged as a result of ice age habitat restriction. Like EF, but in contrast to WHG, CHG carry a variant of the SLC24A5 gene17 associated with light skin colour (rs1426654, see Supplementary Note 6). This trait, which is believed to have risen to high frequency during the Neolithic expansion18, may thus have a relatively long history in Eurasia, with its origin probably predating the LGM. A partial genome from a 24,000-year-old individual (MA1) from Mal’ta, Siberia6 had been shown to be divergent from other ancient samples and was shown by Lazaridis et al.1, using f4 statistics, to have more shared alleles with nearly all modern Europeans than with an EF genome. This allowed inference of an ANE component in European ancestry, which was subsequently shown to have an influence in later eastern hunter-gatherers and to have spread into Europe via an incursion of Steppe herders beginning ∼4,500 years ago5,7. Several analyses indicate that CHG genomes are not a subset of this ANE lineage. First, MA1 and CHG plot in distinct regions of the PCA and also have very different profiles in the ADMIXTURE analysis (Fig. 1). Second, when we test if CHG shows any evidence of excess allele sharing with MA1 relative to WHG using tests of the form D(Yoruba, CHG; MA1, WHG) no combinations were significantly positive (Supplementary Table 6). Last, we also tested whether the ancestral component inferred in modern Europeans from MA1 was distinct from any that may have been donated from CHG using tests of the form D(Yoruba, MA1; CHG, modern North European population) (Supplementary Table 7). All northern Europeans showed a significant sharing of alleles with MA1 separate to any they shared with CHG. WHG and CHG are the descendants of two ancient populations that appear to have persisted in Europe since the mid Upper Palaeolithic and survived the LGM separately. We looked at runs of homozygosity (ROH: Fig. 3) which inform on past population size3,19,20. Both WHG and CHG have a high frequency of ROH and in particular, the older CHG, Satsurblia, shows signs of recent consanguinity, with a high frequency of longer (>4 Mb) ROH. In contrast, EF are characterised by lower frequency of ROH of all sizes, suggesting a less constricted population history20,21, perhaps associated with a more benign passage through the LGM than the more northern populations (see Supplementary Note 7 for further details). |
|









