|
|
Post by Admin on Mar 13, 2021 3:38:08 GMT
Reconstructing Hard Tissues The production of hominin reconstructions is interconnected with the discovery of fossils. This is not surprising since the internal skeleton serves as the basis for all of the external soft tissues. The vast majority of hominin fossils are represented by skulls, which are well-connected sets of bones that are usually preserved together, although often distorted or missing mandibles, unlike postcranial remains that consist of many separate bones that can become easily scattered in the environment (Suzuki and Takai, 1970; Sartono, 1972; Brown et al., 1985; Suwa et al., 2009; Berger et al., 2010; Kimbel and Rak, 2010; Laird et al., 2017). Postcranial fossils, by comparison, are exceptionally fragmented. Large portions of these fossils are poorly represented and/or were never recovered. Therefore, before the soft tissue for any hominin can be considered, the osteological material must first be reconstructed. Methods for the reconstruction of hominin crania have been, and are still being, developed (Kimbel et al., 1984; Kimbel and White, 1988; Zollikofer et al., 2005; Gunz et al., 2009; Suwa et al., 2009; Kimbel and Rak, 2010; Benazzi et al., 2011; Amano et al., 2015; Brassey et al., 2018). In 1984, Kimbel, White, and Johanson reconstructed a male Australopithecus afarensis skull. The skull was a composite reconstruction that incorporated the skeletal elements from 12 different supposedly male fossil specimens found from sediments at A.L. 200-1a and one specimen found at A.L. 333/333w. This skull was later revised after the discovery of further fossil evidence (Kimbel and White, 1988). Similarly, in 1996, Tattersal and Sawyer revised (Weidenreich’s, 1937) reconstruction of the skull of Homo erectus from a collection of casts from Zhoukoudian, China (Tattersall and Sawyer, 1996). This reconstruction was different from the Weidenreich skull, which was reconstructed as a female, whereas Tattersal and Sawyer reconstructed the skull as a male (Tattersall and Sawyer, 1996). To the knowledge of the authors, these are two of the only physical reconstructions of hominin skulls that have had their initial reconstruction and subsequent revision formally published. What this means for all other reconstructions of hominin skulls is unclear. Reconstructions of hominin skulls facilitated by computer software are becoming increasingly popular (Gunz et al., 2009; Benazzi et al., 2011; Gunz and Mitteroecker, 2013; Kikuchi and Ogihara, 2013; Amano et al., 2015; Senck et al., 2015; Mounier and Mirazón Lahr, 2016). Gunz et al. (2009) produced virtual reconstructions of three hominin skulls from CT scans of the original specimens. These were the Taung child skull, the adult specimen of Australopithecus africanus Sts 5 (Broom, 1947), and a skull of the Homo erectus juvenile specimen KNM-WT 15000 (Brown et al., 1985). For the Sts 5 specimen, CT scans were combined with geometric morphometric methods to produce a complete skull. Landmarks were applied to a modern human cranium for the purpose of extracting coordinates and to produce a reference surface. The surface of the original Sts 5 cranium was then warped to match those coordinates taken from the modern human reference. This method goes beyond the reassembly of missing fragments like a jigsaw puzzle, such as those mentioned previously, as the entire fossil is replaced with a warped model of the modern human reference cranium. In other words, no fragments belonging to the original fossil are preserved in the result. For this reason, the method has received criticism (Senck et al., 2015). Accuracy of the method hinges on the correct use of reference surfaces. Interspecies and intraspecies reference surfaces can produce different results. Senck et al. (2015) concluded that it is possible to reconstruct hominin crania using reference surfaces but only if the morphometry of the subject being reconstructed is similar, or if bilateral symmetry can be exploited. When we reconstructed the Taung child’s skull in 2017, we used traditional molding and casting methods to produce a duplicate cast made directly from the first-order cast of the original specimen that was gifted to MH in 1995, rather than commercially available products—such as those from Bone Clones, Inc.—which are not exact copies of the original fossils themselves. The Taung fossil required very little restoration since its preserved parts provided enough anatomical constraints, such as occlusion and articulation, which meant that very few assumptions were needed to obtain complete anatomical information. However, in our reconstruction of Lucy’s skull shown in Figure 1C, the reconstruction process was not as straightforward. Lucy, being the adult female specimen of Australopithecus afarensis and one of the most complete Pliocene hominin fossil skeletons ever found, has been subject to the facial reconstruction procedure more so than any other fossil hominin. By attempting to reconstruct Lucy’s skull ourselves, we found that this specimen is a poor candidate for the facial reconstruction procedure because most of Lucy’s cranial bones are missing. Lucy’s mandible (Figure 1B) is relatively well-preserved and as such formed the basis for our reconstruction, but the cranium had to be digitally interpolated from the previously discussed composite male skull shown in Figure 1A (Kimbel et al., 1984; Kimbel and White, 1988). While doing so we discovered that the male cranium is much larger and does not articulate with the mandible well, so we scaled the cranium uniformly on all axes to fit Lucy’s mandible based on bilateral symmetry and parabolic curve alignment of the upper and lower dental arches. The method we employed can be described as a “best-fit” approach and we do not by any means present our own reconstruction of Lucy’s skull as the definitive version of this individual. However, it does show how each practitioner is required to model their own skull or borrow commercially available products that have never been formally verified. FIGURE 1 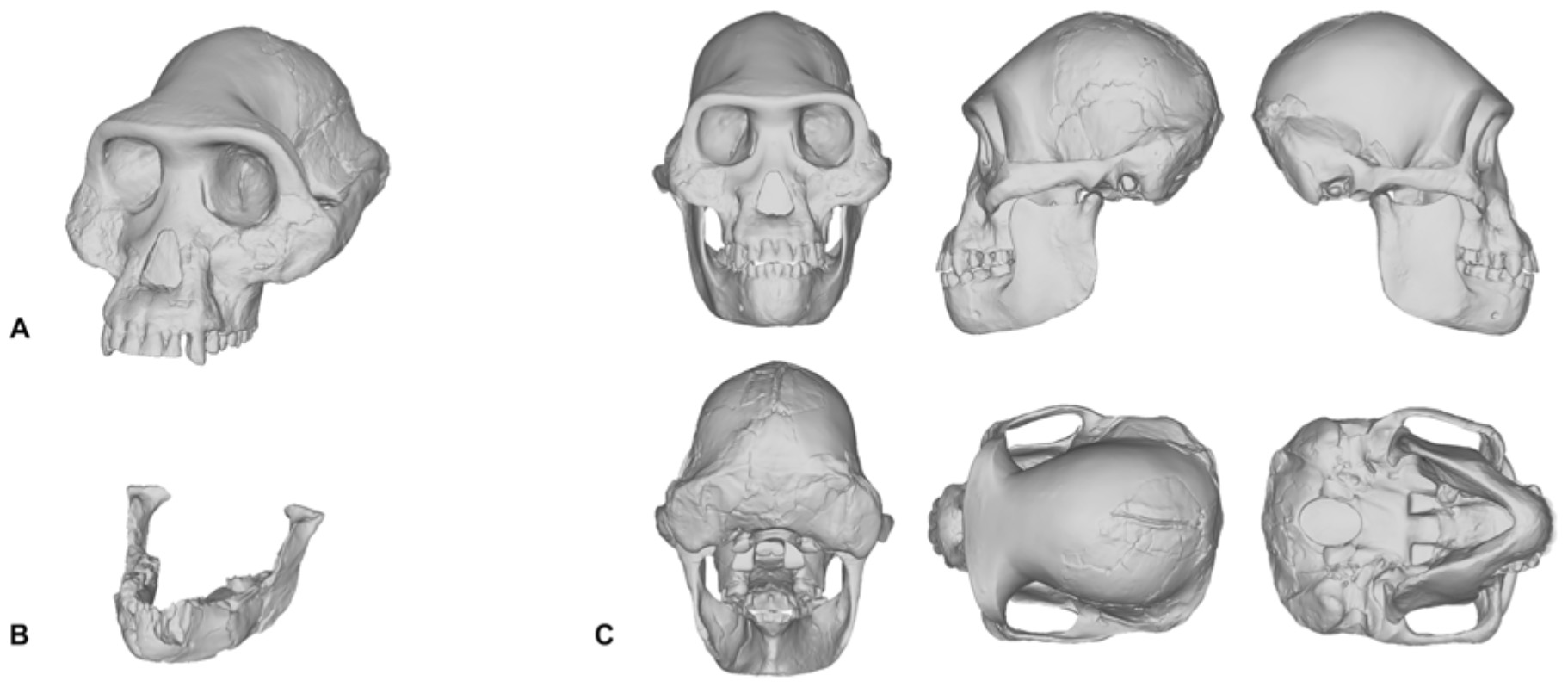 What can be inferred from the methods involved in the reconstruction of hominin skulls is that separate methods are likely to produce varying results, especially in the case of Lucy. There is one other fact that needs to be acknowledged here. Since Lucy was discovered, other better preserved skulls have been found. So well-preserved are these skulls that almost no osteological reconstruction is necessary. The skulls belonging to individuals attributed to Homo naledi (LES1), Australopithecus sediba (MH1), and Homo floresiensis (LB1) are just a few specimens that are ideal candidates for the facial reconstruction procedure (Brown et al., 2004; Berger et al., 2010; Laird et al., 2017). Despite these new discoveries, and to our surprise, there are still facial reconstructions of Lucy being performed today. For these reasons, we would like to encourage practitioners to perform facial reconstructions on well-preserved fossils first before attempting to reconstruct those that are heavily fragmented. Since this special issue is about more than just reconstructions of the skull and face, we feel that it is essential to include the various attempts at reconstructing hominin post-cranial skeletons in this review. However, an extensive survey of the scientific literature revealed that there is only one peer-reviewed article including a reconstruction of a complete hominin postcranial skeleton. The skeleton was reconstructed in a recent study exploring the use of a volumetric technique for estimating the body mass of hominins, in which a complete virtual 3D model was reconstructed for, yet again, Lucy (Brassey et al., 2018). However, in this case the subject is a logical choice since Lucy’s post-cranial skeleton is exceptionally preserved. In this reconstruction, scans were made from casts of the original fossil bones and then virtual reproductions were articulated in computer software. 3D modeling techniques, such as mirroring and sculpting, were then used to reproduce existing parts of the skeleton. Additional hominin fossils were used for the completion of missing parts, including, but not limited to, an Australopithecus sediba (UW88-38) right clavicle and the Homo habilis specimen OH-8. Scans were also made from these elements and the virtual reproductions were then scaled to fit the partial skeleton. The thorax morphology was reconstructed using an iterative, geometric morphometric technique based upon a sample of both Homo sapiens and Pan troglodytes. The resulting 3D model of Lucy’s skeleton was used in our reconstruction of Lucy’s face and body (Figure 2). Putting the soft tissues aside for the moment to focus on the skeleton alone, we are not confident that the 3D model reconstructed in Brassey et al. (2018) is a true representation of Lucy’s anatomy. The decision to reconstruct Lucy as an upright, free-standing hominin fully capable of erect bipedalism is well supported; it is indicated by the anatomy of the A.L. 288-1 fossil, the discovery of earlier and more numerous fossils attributed to Australopithecus afarensis, and the footprints from the Laetoli Beds of northern Tanzania (Leakey and Hay, 1979; Leakey, 1981; Johanson et al., 1982; Kimbel et al., 1984; Aiello and Dean, 1990). The footprints, for example, demonstrate that at the time of the Australopithecus there existed upright, free-standing hominins fully capable of walking bipedally and, therefore, Lucy has been reconstructed in such a way as to make this functionally possible. However, we agree with Brassey et al. (2018) in that the reconstruction is incorrect but only to the extent that the addition of skeletal elements from other specimens—belonging to separate species—will inevitably produce error, and how could it not? One could never confidently extrapolate the missing bones from an anatomically modern human with those belonging to a chimpanzee, so why would the talus from the Homo habilis specimen OH-8 be a suitable substitute for the talus of Australopithecus afarensis? We would also like to add that the ribcage is highly speculative. It is currently held that anatomically modern humans and hylobatids (gibbons and siamangs) share a barrel-shaped ribcage, whereas the great apes share a funnel-shaped rib cage. However, hypotheses about the shape of the Australopithecus rib cage vary and a consensus is yet to be reached on whether Australopithecus were markedly different from great apes and more similar to modern humans, or if the Australopithecus rib cage was more comparable to extant intermediates, such as hylobatids and orangutans (Bastir et al., 2017). Furthermore, the general stance of the skeleton is also potentially in error in part due to the ilium of the pelvis seeming to be angled in a position not seen in any extant hominin, much less a hominid. This horizontal position of the pelvis makes Lucy’s stature shorter since the acetabula are raised upward and forward. Thus, similar to what has been previously discussed regarding hominin skulls, variability among post-cranial soft tissues is not just the result of differences in the shaping of the external appearances, it also appears to be the result of differences in the anthropometrics and arrangement of the underlying post-cranial skeletons. FIGURE 2  Although peer-reviewed articles including reconstructions of postcranial hominin skeletons are lacking in the scientific literature, there have been a number of reconstructions produced and published in books intended for a general audience. For example, in 2013 the skeleton of the Paranthropus boisei specimen OH5 was reconstructed by John Gurche for a display at the Smithsonian Museum of Natural History, and is featured in Gurche’s (2013) book Shaping Humanity. The height of the skeleton appears to have been informed by a regression model developed in Gidna and Domínguez-Rodrigo (2013) and used by Domínguez-Rodrigo et al. (2013) to produce minimum stature of 156 cm for this individual. However, the prediction model was developed using anatomically modern human anatomy, which Domínguez-Rodrigo et al. (2013) concede may not be appropriate in the case of the Paranthropus genus. We would like to highlight that even if the predicted minimum stature was correct, it does not provide the actual height for this individual nor the measurements of specific lengths of long bones. As of today, the only postcranial fossil that has been assigned to this species is the proximal end of an adult left femur. No other postcranial fossils have been confidently assigned to this species. Gurche provides a brief description for how he extrapolated the body from other australopithecine specimens (Gurche, 2013), but the results are highly speculative and virtually impossible to verify without the discovery of postcranial fossils belonging to Paranthropus boisei. The fact is that this reconstruction of Paranthropus boisei really only acts as an ill-informed hypothesis that is largely untestable. This is a notion that not only pertains to skeletal reconstructions of this species but to the practice of hominin reconstruction as a whole. What this rather obviously shows is that we are in desperate need of more fossil evidence, especially since bones serve as the starting point in all reconstructions of ancient primates. |
|
|
|
Post by Admin on Mar 13, 2021 21:34:22 GMT
Reconstructing Soft Tissues Fossilized specimens of soft tissue are exceptionally rare. To the knowledge of the authors only one has ever been found for a primate (Franzen et al., 2009; Lingham-Soliar and Plodowski, 2010). The discovery was described as Darwinius masillae, an Eocene primate that lived 47 million years ago. What is most exceptional about this specimen is the almost complete skeleton, which is surrounded by a dark shadow representing the outline of the body and clearly showing gross anatomical details, such as the size of muscles surrounding the long bones, as well as minute details, such as the size of the external ears. Fossilized soft tissues have been found for other species, such as a specimen of the Cretaceous dinosaur, Psittacosaurus (Lingham-Soliar and Plodowski, 2010), and the Pliocene vulture, Gyps fulvus (Iurino et al., 2014). However, no such material has ever been found for any Plio-Pleistocene hominin species and, given the absence of soft tissue in the fossil record, there is no direct evidence for practitioners to extrapolate the soft tissues from or to compare their results with. Practitioners of facial reconstruction must therefore employ methods developed in studies of anatomically modern humans, which have mainly focused on the face. The foundations for these methods were laid in the nineteenth century by anatomists Hermann Welcker and Wilhelm His (Welcker, 1883; His, 1895). Welcker and His carried out the first documented research on the relationship between skull morphology and the soft tissues of the face by collecting soft tissue depths measurements at nine facial points from European cadavers, of which 37 were male and four female. A facial reconstruction was subsequently performed on a plaster cast of the skull of German composer and musician Johann Sebastian Bach using the measured thicknesses to construct the tissues of the face. This work has been often cited as one of the first facial reconstructions (Prag and Neave, 1997). Another well-known early facial reconstruction was performed by Kollman and Büchly (1898). Kollman and Büchly reconstructed the face of a Neolithic woman from Auvernier in Switzerland. The reconstruction was a joint effort, where Kollman collected soft tissue measurements from hundreds of female cadavers and produced a plan for the procedure and Büchly modeled the tissues onto the skull to produce the face. These early attempts of reconstructing faces to approximate the appearance of the deceased are cited in almost all of the literature on forensic facial reconstruction (Prag and Neave, 1997; Wilkinson, 2004). Today, methods detailing the reconstruction process of the face are ubiquitous in the facial approximation literature (Stephan, 2003a,b,c; Stephan et al., 2003, 2013; Wilkinson, 2004; Hanebrink, 2006; Stephan and Simpson, 2008; Guyomarc’h et al., 2012; Richmond, 2015). Part of the challenge for any practitioner of hominin facial reconstruction is deciding which methods to use since a single anatomical feature may be reconstructed using a number of separate methods. In reconstructing the soft tissues of hominins faces, measurements at various cephalometric landmarks on the face must be determined. There are currently only three methods available to practitioners for reconstructing hominin soft tissues: (1) the thicknesses can be derived from mean values taken from measurements of modern humans—the best resource for deriving mean values comes from a recent meta-analysis of all the data drawn from across all of the literature (Stephan, 2017)—(2) the thicknesses can be derived from regression models developed from measurements of modern human skeletons and corresponding soft tissues, or (3) the thicknesses can be derived from mean values taken from measurements of great apes (chimpanzees, gorillas, and orangutans). There are a few recognized reasons why mean values derived from either modern humans or apes, especially chimpanzees, may not be appropriate for reconstructing the face of Plio-Pleistocene hominins. First, means only express averages and thus do not represent the reality of individual variation within populations and, in fact, they completely ignore it. Furthermore, extrapolation of modern human depth data to archaic hominin skulls like those belonging to robust Australopithecus, such as the OH5 specimen, is predicated on the assumption that soft tissues depths between separate hominin species are identical, which is false based on what soft tissue measurements have been taken from chimpanzees (Hanebrink, 2006), and while extrapolation of mean chimpanzee values may produce less error than those for modern humans, very few measurements have ever been obtained for chimpanzees and therefore much of the face is still subject to artistic interpretation. For the above reasons, we rejected the use of averages in our own reconstructions. Conversely, the use of equations for predicting facial tissue thicknesses from craniometric measurements is gaining traction (Sutton, 1969; Simpson and Henneberg, 2002; Dinh et al., 2011; Stephan and Sievwright, 2018). Multiple significant correlations have been identified in samples of modern humans and regression models have been produced. As such, craniometric measurements of the skull can be used to produce facial tissue depths from regression models alone. Given that the soft tissues are tailored to each skull and are based on the verified relationships between soft tissue and craniometric dimensions, this method ought to be explored further, especially in great ape material, for the possibility of producing a set of regression models that have inter-species compatibility could reduce most of the variability between facial reconstruction of the same individual. In our own experiments, results varied depending on whether intuition or equations were used. Given that practitioners of hominin reconstruction have chosen not to publish their methods it is not possible to link methods to any given reconstruction for the sake of comparison, so here we can only analyze our own facial reconstructions as a means of exploring the strengths and weaknesses of each method. To do so, we point to our reconstructions of the Taung child. The first reconstruction was produced using GV’s sculptural and anatomical intuition alone, while the second was produced a year later using the same method except under the supervision of MH. As can be seen in Figure 3, there are obvious differences in their appearance. If intuited reconstructions that are produced by the same practitioner can vary, in particular with input from outside sources, then one can see clearly why reconstructions of the same individual produced by separate practitioners could vary wildly from museum to museum. FIGURE 3 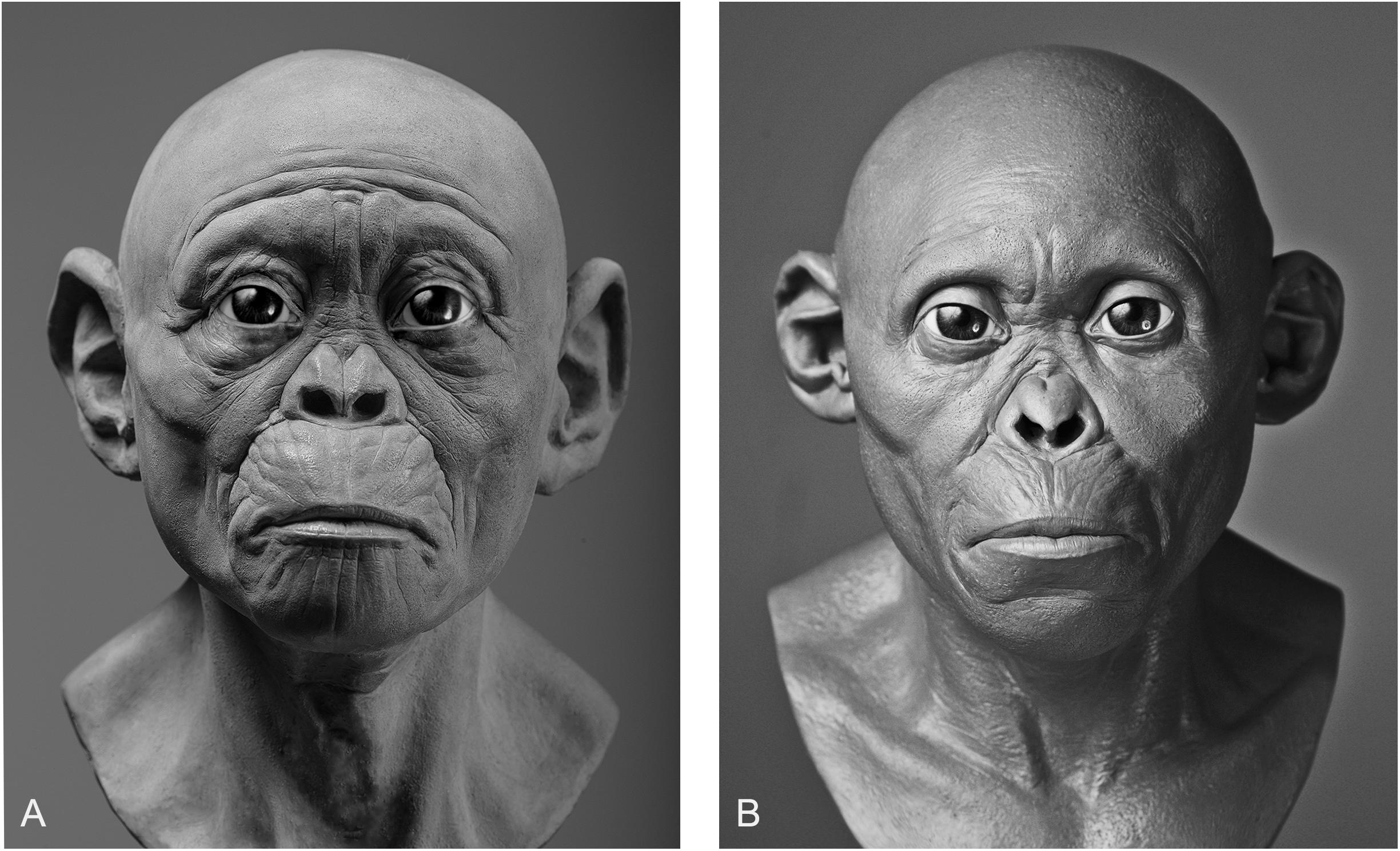 There are also other aspects beyond soft tissue thicknesses at specific points on hominin skulls that affect the variability exhibited between reconstructions of the same individual. The placement of the eyeballs within the orbits, eyebrow position, mouth width, and ear size arguably have more of an impact on the appearance than soft tissues alone. Much like the soft tissue thickness, these features have been either reconstructed intuitively or using methods derived from studies of anatomically modern humans and great apes. In Gurche’s reconstruction of the Australopithecus africanus specimen Sts-5, Gurche reconstructed the mouth width based on measurements of Pan troglodytes (Gurche, 2013), and eyeball position based on an unspecified ratio described in the appendix of his publication. In our reconstruction of the Taung child, we found that if official methods were not followed the reconstruction could be made to appear in a number of different ways. The mouth of the reconstruction in Figure 3A appears more prognathic than the reconstruction shown in Figure 3B. The ears are also larger and the flexure wrinkles more pronounced, which is more akin to young bonobos than to modern humans. In hindsight, it appears a concerted effort may have been at play to depict the subject as more ape-man (A) in one case and more man-ape (B) in the other. In an effort to move away from intuition, our second facial reconstruction of Lucy (Figure 4) used equations derived from regression analyses of anatomically modern humans (Simpson and Henneberg, 2002). As one can see, it differs in appearance from the earlier reconstruction of Lucy in Figure 3, which was done intuitively without empirical data. This reconstruction may be perceived as an improvement over the previous Lucy since an empirical method was used, however, we believe that this is not at all the case. We must be fully transparent in stating that a number of the predicted values produced by the regression model yielded negative results, i.e., tissue thicknesses below 0.0 mm. Since it is not possible for soft tissue to be negative or equal to zero, these landmarks were excluded from the reconstruction and instead were extrapolated from the nearest relative predicted value. This error is likely a result of extreme values of the independent variable. While some points did seem to conform to biological reality, based on mean comparisons, the fact that some points were entirely outside of possibility should cast doubt on the entire efficacy of human-derived regression models for reconstructing facial soft tissue in australopithecines. Thus, these equations are perhaps only appropriate for reconstructing hominins with craniometrics that are inside the normal range of variation observed in samples of anatomically modern humans. FIGURE 4  |
|
|
|
Post by Admin on Mar 14, 2021 0:37:20 GMT
Our reconstruction of the Neandertal specimen Amud 1 (Figure 5), for example, exhibits less of the aforementioned issues. The more proximal relationship of Neandertals to modern humans makes the use of the equations more viable. A number of other empirical methods derived from modern humans were also used, including positioning the eyeballs according to Guyomarc’h et al. (2012), the profile of the nose according to Prokopec and Ubelaker (2002), and the width of the mouth according to Stephan and Henneberg (2003). The final facial reconstruction of Amud 1 shown in Figure 6 is similar to other reconstructions of Neandertals, especially in the size of the nose, suggesting that there is less variability in individuals that are compatible with existing methods of facial reconstruction derived from modern humans, although an explanation for this compatibility remains unclear. It is important to note here that while no values were reported as negative, unlike in our facial reconstruction of Lucy, we think the lack of lateral points on the skull offered by the equations resulted in too much intuition at these areas. This is because facial reconstruction methods have focused only on points of the face for the purposes of identification, whereas differences in the appearance between species can extend beyond the face to the whole head, like the temporalis muscles of OH5 for example. Thus, a more comprehensive study involving more measurements and points around the entire skull warrants further investigation. FIGURE 5 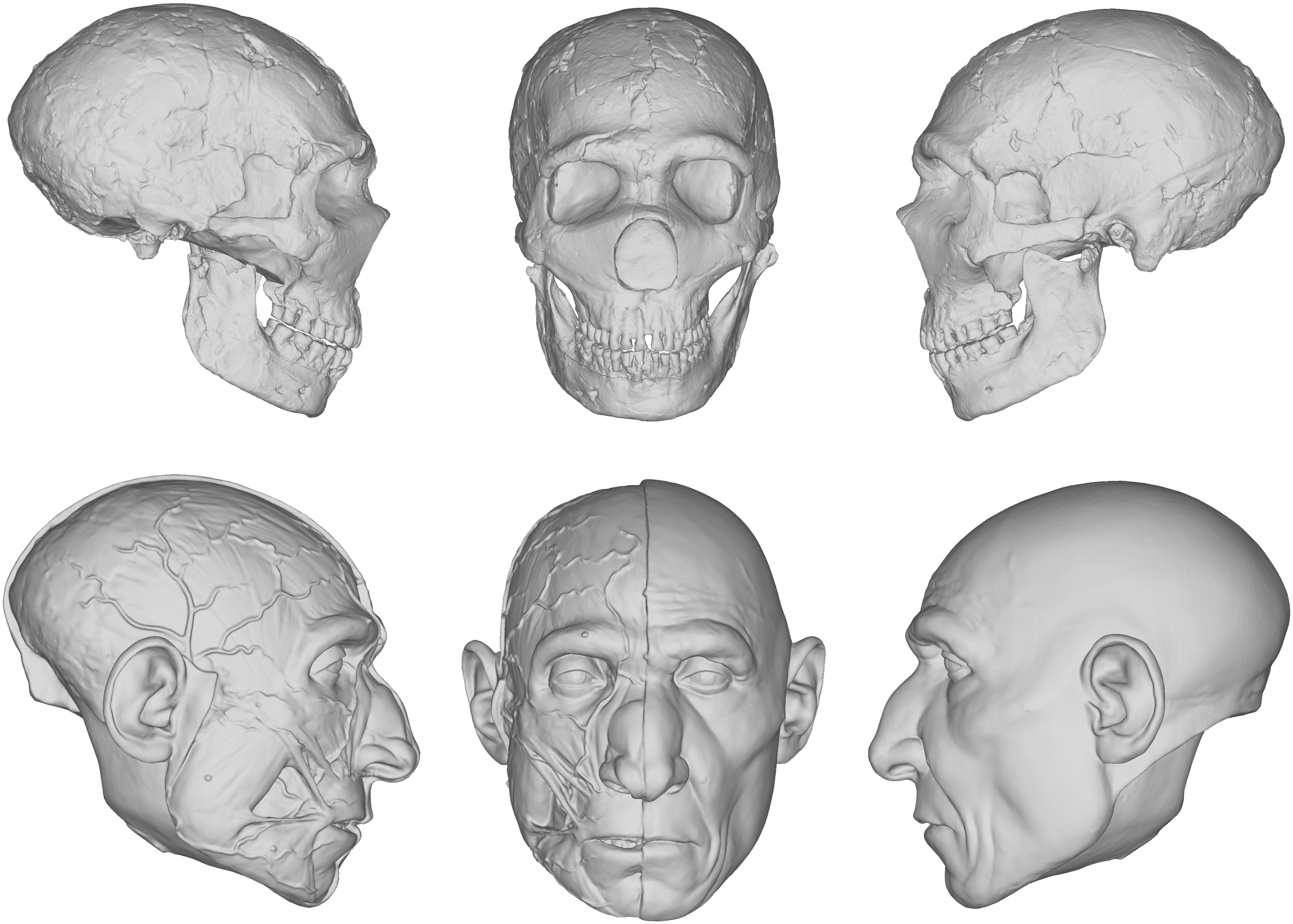 Digital models showing the progression of the facial reconstruction procedure. Subject is based on a reconstruction of the Neanderthal Amud 1 cranium and associated mandible originally reconstructed by Suzuki and Takai (1970). Facial soft tissues were reconstructed using regression models developed in Simpson and Henneberg (2002) from modern human material. FIGURE 6  Facial reconstruction of Amud 1 (without hair and pigment) produced in 2019 that employed facial soft tissue regression models developed in Simpson and Henneberg (2002) from modern human material. Regarding soft tissue reconstructions of hominin bodies, the only published method we could find is described by Gurche (2013). This method, which has no empirical basis, was used to reconstruct the body of a number of Plio-Pleistocene hominins. We used the same method in our reconstruction of the body of Lucy in Figure 2. Like Gurche, we inferred the muscle proportions from comparative studies of fossil hominins and great apes. One of these studies reported that the ulnae of A.L. 288-1 have short, proximally oriented olecranons, whereas all great apes have long distally oriented olecranons (Drapeau, 2003). This difference in olecranon morphology is reported to be the result of different functional requirements. The long olecranons of the ape ulnae reflect powerful triceps brachii muscles adapted for arboreal use, whereas the short olecranons of A.L. 288-1 reflect triceps brachii muscles adapted for manipulative activities, such as tool making (Drapeau, 2003). Thus, in our reconstruction of Lucy’s body, we reconstructed the upper limbs to reflect the functional predictions we could extrapolate from the ulnae. Unfortunately, comparative studies such as those described are lacking for the trunk and lower limbs, so these are highly speculative and subject to change. As a whole, we found that the intuitive method for reconstructing the soft tissues of hominin bodies far too imprecise. Another point of contention is skin color, which is the single most under-researched feature in relation to hominin reconstruction and there is no known method for reliably reconstructing skin color in hominins. In modern humans, mass migration has made it impossible to predict skin color with any precision. This is mainly due to interbreeding and mismatches between the ancestral environments that shaped our appearance and the environments we inhabit now (Jablonski, 2013). This is perhaps the reason why no effort has been made to develop a method for reconstructing skin color in ancient hominins. The consequence of not having a method for determining the appearance of hominin skin is illustrated in the varying reconstructions produced by Gurche, Daynès, Sawyer, Deak, and the Kennis brothers, as well as in our own reconstructions. As can be seen in the completed facial reconstructions of the Taung child (A) and Lucy (B) presented in Figure 7, their skin tones differ significantly. We have interpreted this difference as a result of not having an empirical method for reliably reconstructing epidermal melanin concentrations in australopithecines. The color of the Taung child’s skin was reconstructed to appear similar to modern Homo sapiens native to Southern Africa. The decision to reconstruct the skin in this way is based on what is known about the function of epidermal melanin. Melanin evolved as a physical and chemical barrier to filter ultraviolet radiation. In humans there is a strong relationship between latitude and skin color and variation in skin color is the result of differences in concentrations of melanin (Blum, 1969; Relethford, 1998; Barsh, 2003; Chaplin, 2004; Jablonski, 2013). High concentrations of melanin are evolutionary advantages for populations in close proximity to the equator because it is the optimal arrangement for ultraviolet filtration in that environment. We assumed that for the Taung child to survive in Southern Africa there would have been no advantage in having low concentrations of melanin. Indeed, since it would have been a disadvantage and since ultraviolet radiation is the only known selective pressure for evolutionary change in melanin concentrations, we inferred that the skin of the Taung child would have been dark in appearance. However, even if this assumption is true, Lucy was reconstructed using exactly the same logic, although the results are very different. The appearance of the skin may be altered based on one’s own subjective interpretation of the taxonomic position of these specimens. Both the African great apes, such as gorillas and chimpanzees, and modern humans have dark skin but “dark” is not nearly as descriptive as one may initially think. Regression models for reconstructing skin tone have been developed in Jablonski and Chaplin (2000), however, they measured melanin concentrations by skin reflectance, which does not provide the practitioner with a visual representation of the skin color of the subject. Research in this area offers the opportunity to present hominin populations with melanin concentrations that actually match their ancestral environments. FIGURE 7  The color of primate pelage and differences between species further complicate the process of reconstructing surface appearance in hominins. For our reconstruction of the Taung child and Lucy presented in Figure 7, each hair was individually implanted into silicone casts of the reconstructions using a crown punching needle following the direction of hair in Homo sapiens and great apes described in Kidd (1903). We found that pelage was the most challenging feature to reconstruct because the pattern and distribution of hair cannot currently be extrapolated from bone alone. We tried to follow current hypotheses regarding thermoregulation via exploitation of exocrine sweating, which is often cited as a potential influence on the evolution of hairlessness in Homo sapiens (Wheeler, 1991, 1992), however, these hypotheses do not provide a current phenotype for specific species. Even considering further hypotheses about how hairlessness evolved from spending more time in aquatic environments (Hardy, 1960; Morgan, 1997), and in order to free our ancestors from external parasites (Pagel and Bodmer, 2003), neither of these explanations provided us with the specific instructions required to determine hair color and density. For all of these reasons, pelage poses a problem for museum displays. It has been said that baldness is preferable in an evidence-based reconstruction (Hayes et al., 2013). We do not necessarily agree with this as any reconstruction without hair may be perceived as incomplete or suggest that hominins did not have hair. This does not mean that we advocate for imaginary speculation in this area merely for the purpose of completing the reconstruction, rather, we would strongly encourage further research in this area. Future Research The detection and analysis of DNA in extinct hominin finds is an emerging field and offers the exciting possibility of greatly enhancing reconstruction methods. Today, genetic research relevant to the practice includes the following: comparison between the genomes of Pan troglodytes, Pan paniscus, and Homo sapiens has revealed similarities between species and has enabled scientists to reconstruct the ancestry between them (Prüfer et al., 2012); the DNA of the Neandertals has been sequenced from a 38,000 year-old-fossil that was free from contamination with modern human DNA (Green et al., 2006), which has made it possible to compare the Neandertal genome to that of modern humans; and lastly, efforts to reconstruct the skeletal anatomy of the Denisovan’s using DNA analysis generated body plans for these archaic hominins (Gokhman et al., 2019), as noted in the introduction, although the results are far from certain. Due to the chemical structure of DNA molecules, it is unlikely that they will preserve for more than several scores of thousands of years, thus there is little hope to obtain DNA of Pliocene/Early Pleistocene hominins. Proteomics seems to be able to study aminoacid sequences in ancient bones of greater antiquity since molecular structure of polypeptides preserves better than structure of DNA. However, genetics does not currently provide the precise measurements needed for the reconstruction of both hominin soft tissues and underlying bone structures. The morphology of the bones in the illustration showing the Denisovans body plan is highly subjective (Gokhman et al., 2019). There is currently no known method for deriving anthropometric measurements from genomes, highlighting a major problem with the proposed body plan. The main purpose of the illustration appears to be providing an example rather than a precise depiction of anatomical forms from the past. Therefore, it seems that anatomical data are best provided by direct observations of anatomical structures. There is the possibility that genetic research will provide information about hominin appearances that cannot be determined from bone alone. Eye, hair, and skin color are just some aspects of hominin appearances that may be determined from the sequencing of ancient hominin genomes. Unfortunately, this information will be restricted to specimens from the late hominin record (Neandertals, Denisovans, and LB1) because, as stated, DNA extraction is not possible from fragments that are older than a few hundred thousand years. Worse still, DNA extraction from fossils is impossible. Fossils are bones that have all organic compounds replaced by minerals from soil and do not contain DNA. Alas, the only hominin remains that will be available for genetic research will be those that are not fossilized. |
|
|
|
Post by Admin on Mar 14, 2021 4:14:13 GMT
The Ethics of Reconstruction and Societal Implications Given what Anderson (2011) has shown regarding the variability present in reconstructions of the same individual across separate museum displays, it is clear that very little effort has been made to produce reconstructions that are substantiated by strong empirical science. This is surprising given how museums boast about decades of success presenting scientific knowledge and education to the public. While in large part this is true and they provide an invaluable service to society, with respect to hominin reconstructions, they appear to exaggerate the methods used or this information is left out of their displays entirely. The reasons for this are not certain so we can only hypothesize as to the reasons why. It can most likely be attributed to factors outside the control of science, namely economic and social concerns. The immense pressure for museums to produce exhibits that are exciting may get in the way of any efforts to present reconstructions that are based on actual scientific knowledge, which requires time and effort. Exciting exhibits that feature large and very complete objects may attract non-traditional audiences, whereas small exhibits that grow over time presenting what is actually known about the appearance of Plio-Pleistocene hominins may only be of interest to a narrow audience. Museums are often hubs for scientists and educators to share ideas with each other and find practices to excite the public with their enthusiasm. Truly, despite our criticisms, we acknowledge that generations of learners of all ages, educators, artists, and all forms of curious people have benefited greatly from the existence of museums. However, presenting information that is not known diminishes the value of that which is known and may lead to confusion and discourage further interest in human evolutionary theory. There are potential educational harms in presenting unscientific reconstructions of hominins under the shroud of presumed validity. Therefore, with the cultural role of a museum and any educational institution, or any educational tool at all, comes an added responsibility to take pains to avoid accidentally, or worse, willfully misinforming the public. While institutions showcasing and not challenging these empirical errors is troubling, other errors less concerned with what hominins looked like can be potentially far more damaging to social perceptions of evolution and its implications. To explore this point, it is important to introduce a couple of terms and a sentiment from an artistic perspective. For the academic art community, understanding iconography and iconology when creating representational works is crucial. In the visual arts, iconography is the study of subject matter itself and iconology is an attempt to analyze the significance of that subject matter in relation to the culture and individuals that produced it. This distinction is important because depictions of hominins do not exist in a vacuum, rather they are seated in the historical contexts of not just science but also those of the arts and cultures. This issue has been discussed in many books, including Moser’s (1996) Ancestral images: the iconography of human origins, which analyzes how biases, prejudices, and stereotypes had been crucial in such reconstructions and further reinforced by them. Therefore, like an institution can be held accountable for what it promotes and showcases to the public, artists too can be held accountable for how they represent their subjects in their artworks. In Van Laar and Diepeveen (1998) the roles artists function under within society are explored. One of these roles is that of the artist as an intellectual. This role is exemplified as the artist who deals with areas of human knowledge and contributes to them; the paradigmatic career of Leonardo Da Vinci comes to mind as the example that fits this mold. The tradition of artists working within the disciplines of science has undoubtedly contributed to scientific knowledge. As such, it can easily be argued that artists working in the field of hominin reconstruction operate under a similar role. However, as Laar and Diepeveen point out, with the obvious benefits to this role comes the danger of elitism being exercised by the artist. Artists tend to get self-absorbed in their claims about art and culture, making artwork that is seldom understood by the public and often disagreed with by art professionals. In other words, what begins with a sincere interest to contribute to human knowledge can become an ideological arms race in a competitive art field regarding the insights of individual artists who constantly jostle for artistic relevance. While this point is being made within the context of the art world, this same danger is present in the field of hominin reconstruction. Artists who are commissioned to sculpt models for museums tend to be highly skilled in the sculptural arts and their interest to contribute to science is at times overshadowed by what they can do artistically. Like the ideological arms races of heady conceptual artists, the museum display circuits can also be subjected to a similar form of competition. They can be so much more concerned with making science exciting that they can forget the underlying mission of their role in this context, which is to disseminate and contribute to actual scientific knowledge. Artists who purport to facilitate the dissemination of scientific material, whose works are also hosted by renowned institutions of learning, are understandably perceived by the public as experts in their field. However, when artists operating as disseminators of science fail to make sure their models showcase the best available evidence they fall short in their role of not just educators but artists as well. When work is being consumed by the public for scientific understanding, that status comes with immense responsibility and accountability. Throughout history, people of all ages have looked to artists for inspiration, contemplation, and in many cases like the ones in question, information. Artists who do not take into account or even exploit their contextual roles are at risk of doing society a disservice. For example, consider the most iconic image of human evolution: Rudolf Zallinger’s The March of Progress, also known as The Road to Homo sapiens. Gould (1989) was the first to point out the flaws in this reconstruction, which perpetuates a number of misleading, and potentially harmful, ideas about human evolution. First, it presents the erroneous view that evolution entails a linear progression from animal to ape, to ape-man to the so-called “Negroid race” and then to the “Caucasoid race.” This Euro-centric bias not only makes biological errors but also projects ethical insensitivities. Note that the Zallinger’s image was printed in a series of Science books for public consumption in America in 1965 at the height of the civil rights movement in a country wherein people were afforded different sets of rights and often denied basic freedoms, all based upon variations in skin color. Based on the pernicious bias out of which this image was made, it is hardly appropriate to use it for disseminating scientific information about human evolution. However, imagery of this kind is still being used today. In a promotional video advertising Gurche’s reconstructions present at the Smithsonian Museum of Natural History1, the same errors are present. It shows a linear progression through evolutionary time, transitioning from one genus to the next from Sahelanthropus tchadensis to Australopithecus to Homo erectus, to Homo heidelbergensis to a Neandertal and then finally to Homo sapiens represented by a photo of Gurche himself, who is of European ancestry. Visual material of linear simplified progressions of this sort, even if accidental, can act as a tone-deaf reminder of the history of Europeans holding a place in academia dictating to minorities where they come from and often where they stand in this unscientific hierarchy. This is perhaps most easily seen in the history of art museums and natural history museums housing art in a segregated manner. As expressed in Stanish (2008), art museums have historically showcased the art of the European masters, whereas natural history museums housed the art of indigenous peoples. This Eurocentric myopia has the effect of alienating minorities by putting their artwork in the context of natural history; the domain where we observe the natural world as a separate entity from it. Conversely, the art museum is the domain of artistic achievement. The act of segregating minority culture’s artworks to the building where we study animals is akin to only representing African bodies as a steppingstone on the progression of evolution behind the European body. It may not sound like a point of scientific relevance, but in the field of visual arts ones audience, content, and context are inextricably linked. Artists who show imagery that has relevance to the very identity of our species should be well versed in the troubling iconology surrounding these types of imagery. If education and dissemination are the aims of museums and textbooks, then an extra level of care should be employed in not just what we depict, but how they are depicted and an intimate understanding of who the audience is. Consider how young, would-be academics of minority groups feel as they are readily encountered by not just unscientifically substantiated material, but material that echoes a history of racist attitudes toward groups that look like them. One could understand how visual material of this sort can discourage interest in science. It is important to note here that it is not the intention of the authors to discourage artistic expressions of scientific ideas. If anything, we whole-heartedly support such explorations. As previously noted, artists have held various roles in society and often operate as an inspirational force that can inspire new perspectives outside of the purview of more methodological domains like science. To expand on this point, an artwork by one of us (GV) is presented in Figure 8. Shown is a work inspired by the artist’s involvement within the sciences while employing formal and conceptual cues from art history to explore ideas of identity, origin stories, and even use the formal elements of the veiled cloth as a metaphor for how much is yet to be unveiled about the appearances of our ancestors and evolutionary history as a whole. This work, and other artwork involving the depictions of scientific ideas and/or specimens, serve to invoke thoughts, emotions, and concepts that are of a socio-political and philosophical nature. Thus, works like this have domains in which they are more or less appropriate. Within the domain of the contemporary art gallery or art museum, the scientific inaccuracies or artistic choices are of little consequence since the context puts more weight on the work’s philosophical implications. Conversely, picture for a moment this statue, labeled as an artistic rendition of Lucy, in a natural history museum. Unless there are clear plaques and context-giving aids revealing that the body and its proportions are speculative, and that the use of cloth is a conceptual artistic freedom, this statue would surely mislead adult and, especially, younger museum goers due to the museum-imposed context of education and trust. As the opportunity for confusion outweighs the possibility of education, the prospect of such a work in a natural history museum is perhaps an inappropriate context barring exceptional caveats. Yet there may not be a need to draw such a dichotomous view; if the statue served as an entrance piece that primes the viewers to think about how much we do not yet know, and how heavily veiled the truths about our past are, it can begin a healthy dialogue about what the rest of this imagined exhibit may present to its visitors in the way of fossils and other remains. This is but one example of a way of an artistic object exercising artistic license can operate in an educational context. Yet, this kind of conceptual artistic license is not the one usually taken in museums of natural history, instead practitioners of reconstructions take scientific license and create works much less founded on science than the museums prop them up to be. This is a case of a dim use of the word “art” and “license” operating as handwaving to simply allow artists to fill in the massive gaps in the available evidence with their “vision” without being honest with the public that they are engaged in highly speculative representation. The issue then becomes one of transparency, wherein exhibits could (and perhaps should) take care to show viewers the very exciting and wonderful facts we have uncovered and how much more we do not yet know. This would make what is shown in exhibits scientifically relevant and not inadvertently (or worse purposefully) making claims through their exhibits that are unfounded scientifically as previously discussed. Not taking full account of the context and role both the artist and museum serve together in the aims of scientific dissemination in society can have an adverse effect on the ability of these institutions to fulfill their self-stated aims of societal outreach and education. FIGURE 8  Therefore, models, illustrations, and videos published by reputable institutions and trusted names like the Smithsonian Museum of Natural History should be held to a similar level of scrutiny as papers published in peer-reviewed journals. This is justified given the quantity of daily visits to museums around the world and the amount of visual consumption of content from museum displays, their websites, and printed material, which is far more accessible to the general public than any scientific article. For these reasons, scientists, artists, and museum curators involved in reconstructing our evolutionary antecedents must be very conscious of their role in society as arbiters of scientific facts and the consequences of not conforming tightly to this responsibility. These institutions are ones with a long history of community outreach which have no doubt touched many lives for the better, the authors included. These places have long served as a space where people come to learn and be exposed to not just science, but also to its questions and complexities. Where facts about hominin appearances are unknown, institutions can look to highlight the process of scientific discovery and be transparent instead of relying on artistic liberties and interpretations. Where interpretations or artistic speculation is undertaken, appropriate caveats and information should be readily offered until further research improves on these assumptions. While reconstructions currently displayed in museums globally are impressive for their technical achievement, their lack of scientific foundation paired with an overstatement of their scientific validity may undercut the trust of the public and betrays the very responsibility of dissemination that is expected from such spaces of potential learning. Conclusion The choice of hominins as a case study for this introductory paper of this special issue on muscle reconstructions is due to its value for broader discussions on such reconstructions and on both their ethics and societal implications. Muscle reconstructions are not only of interest to, and used by, scientists, rather they are used in art, textbooks, the press, social media, museums, schools, universities, and many other institutions. That said, the practice of hominin reconstruction has been mostly disregarded as a scientific activity and consequently has not been held to the same standard of scrutiny as peer-reviewed research, despite how the practice is currently perceived. The practice has essentially fallen into the hands of artists who, with no scientific framework of methods yet established for the reconstruction of Plio-Pleistocene hominins, performed the procedure however they wished. Some artists have relied mostly on their intuition regarding the soft tissues, while others have employed the use of forensic facial approximation methods generated from studies of modern human material. However, highlighting such complexities and difficulties also allows us to be aware of the fascinating opportunities that we face: it is a real opportunity for science to offer an alternative and to develop the practice of hominin reconstruction from one that is mostly an artistic activity to one that is a strong empirical science. The question of whether the aforementioned is worth exploring in science seems to be mostly a matter of subjective opinion. Here, the authors would like to propose that no argument can be made against its exploration. Surely, if there is even the slightest evidence to suggest that the practice may improve, then exploration and growth in this area should be encouraged rather than dismissed. Hominin reconstructions are predominately used for the dissemination of scientific information to the public in museum displays and students in university courses, which will influence the way we perceive our common origins, our fellow human beings, and the way we perceive and define humanity more generally. Thus, biologically accurate reconstructions built upon strong scientific foundations will be a non-trivial improvement that will enhance their efficacy and have a positive impact on the public understanding of evolutionary science; a branch of science concerned with our own ancestors and history. This underscores our responsibility regarding their depiction and dissemination because regardless of whether it concerns apes, monkeys, earlier tetrapods, or earlier fish, they are all our evolutionary relatives in the ever-branching biological tree of life. |
|
|
|
Post by Admin on Apr 6, 2021 0:44:55 GMT
 The Kalahari is a huge expanse of desert in southern Africa, stretching across Botswana and into the northernmost part of South Africa’s Northern Cape province. It’s in the Northern Cape that we studied and described a new archaeological site, Ga-Mohana Hill, for research just published in Nature. Our international team, made up of researchers from South Africa, Canada, the UK, Australia, and Austria, has found evidence for complex symbolic behaviors 105,000 years ago. Humans use symbols as a shortcut to communicate important ideas. Identifying the ancient roots of symbolism is limited to what preserves over time. Large calcite crystals from several kilometers away were found in the cave alongside stone tools. Why the crystals were brought there is unknown; they are not modified and do not seem to have a functional purpose. They may have been collected for their aesthetic properties, or included in ritual activities. Crystals are collected by many people around the world to this day for ritual purposes. Early humans bringing crystals into Ga-Mohana suggests innovation in how people interacted with each other and their environment. But such ancient innovation didn’t occur in a bubble: there is context to when and where innovation occurs. What brought people there in the first place, at that time, to begin using those tools and collecting those crystals? Reconstructing past environments allows us to understand this context. And so, a major part of our research centered on working out what the area’s climate was like 105,000 years ago. To do so, we looked at Ga-Mohana’s rocks. The southern Kalahari is often considered too arid to be important for human evolution. Our work contradicts the idea of an arid and empty interior. At some points, Ga-Mohana was much wetter than today, with pools of standing water and waterfalls tumbling down the hillside. The fact that the climate was very different then opens up possibilities about why this previously under-appreciated region must have played an important role in our species’ evolutionary history. Archaeological and geological fieldwork allowed us to piece together this story. The rocks Some kinds of rocks preserve traces of the past environment. The Ga-Mohana hillside is draped in deposits called tufa; these form from water leaking out of cracks in the bedrock. This occurs when underground aquifers are recharged with rain water and begin to overflow. Over time, these waters precipitate calcium carbonate and form tufa. The tufa system is no longer active, apart from small drips during the rainy season. But the fossil tufas represent periods in the past when there was more water available. Similar structures are growing today at places like Sitting Bull Falls, New Mexico in the US. Knowing when the tufas formed at Ga-Mohana tells us when it was wetter there. To find out how old the tufas are and when these wet periods occurred, we used a method called uranium-thorium dating. Uranium is radioactive, meaning that it decays at a constant rate over time and produces ‘daughter’ elements; thorium is one of them. When tufa forms, uranium is ‘locked’ into the crystal structure and begins to decay to produce thorium. The uranium-thorium system acts like a clock that starts when the tufa is formed. By precisely measuring how much uranium and thorium is in the tufa today, we use the known decay rate to calculate when the ‘clock’ started. This method is routinely applied to cave deposits like stalagmites and flowstones but has not been used very much on tufa. This is because dating tufas is not straightforward. Unlike protected caves, tufa forms in the open where sunlight, dust, and debris can contaminate the ages. It took several years of dedicated work to get around these problems: we chose the tufa samples in the field with care and used a sensitive laser to make images of the layers with the most uranium present. We could then target these layers for dating. This provided a real breakthrough. In the end, we dated two layers from an ancient tufa waterfall to between 110,000 and 100,000 years old. This means that fresh water was flowing down the hillside at exactly the same time that people were living at the shelter. Such wet conditions at this time were unexpected, so we wanted to know what caused such a large increase in water to begin with. To understand the reasons for the region being much wetter 105,000 years ago than it is today, we looked at how climate processes influence modern rainfall there. We did this by comparing historical rainfall records to current major climate drivers. We then looked back into the past and used data from an ocean core (deep sea sediments drilled out of the ocean floor which record changes in the earth’s ocean and climate). These data show that parts of the Indian Ocean were warmer around 105,000 years ago. Climate systems are complex, but basically this would have increased the amount of rain in the southern Kalahari, filling the aquifer, and causing the build up of the tufa during this time period. An important role People were drawn to Ga-Mohana for many reasons. Surface water would have been one. The many ostrich eggshell fragments we also found were probably used as water carriers 105,000 years ago. Perhaps these were being filled with water as it flowed down the hillside. One possibility is that water carriers allowed our ancestors to travel further distances. There is still more to be learned from Ga-Mohana, its artifacts, and its rocks. This will allow scientists to understand the role this space played in human evolutionary history better. |
|











